b College of Chemistry, Shahrood University of Technology, Shahrood 36155-316, Iran;
c Faculty of Chemistry, Bu-Ali Sina University, Hamedan 65178-38683, Iran
1. Introduction
Hydroquinone redox systems have been extensively studied because of their biological and environmental importance, as well as the classical fundamental significance of their red-ox chemistry [1]. Hydroquinone has been widely used for the production of dyes, photostabilizers, cosmetics, pesticides, pharmaceuticals, used as photographic developer and an intermediate in the synthesis of antioxidants and polymerization inhibitors [2,3]. So far, hydroquinones have been used as a 1,4-Michael acceptor in the presence of different nucleophiles such as o-phenylenediamines [4], pyridine [5], 3-hydroxy-1H-phenalen-1-one [6], malononitrile [7] and 2-phenyl-1,3-indandione [8]. Thiouracil and some of its derivatives such as propyl- and methylthiouracils which are among the best nucleophiles that could be used in Michael addition reactions. These compounds have some properties such as being minor components of transfer RNA, anti-herpes virus activity and antihyperthyroidism. These compounds reduce the amount of thyroid hormone secreted by the thyroid gland and can control the disease [9].
Regarding the biological activities of hydroquinone and thiouracil derivatives, it is expected if these compounds could be incorporated into on one framework, their biological properties could be enhanced. To the best of our knowledge, the electrochemical synthesis of these compounds has not been reported in the previous literature. In the present work we developed a simple electrochemical method for the synthesis of new hydroquinonethioethers by the electrooxidation of hydroquinone in the presence of 6-propyl-2-thiouracil and 6-methyl-2-thiouracil in aqueous medium. 2. Experimental
Cyclic voltammetric experiments were performed using a MetrohmVoltammetric Analyzer Model 757 (Herisau, Switzerland) and controlled-potential coulometry was performed using a Behpajooh model 2062 galvanostat/potentiostat (Isfahan, Iran). The working electrode (WE) used in the voltammetry experiments was a glassy carbondisk (GC,2mmdiameter)andplatinumdiskwas used as a counter electrodes (CE). The WE used in controlledpotential coulometry was an assembly of 5 carbon rods (6mm diameter and 8 cmlength), and a sheet platinum(1 cm2) constituted the CE. The WE potentials were measured versus Ag/AgCl. All electrodes were from AZAR Electrode Company (Urmia, Iran). NMR spectra were recorded on a Bruker DRX-300 Advance Instrument (Germany). Infrared (IR) spectra were recorded on a Shimadzu 8400S Fourier transform (FT)-IR spectrophotometer (Tokyo, Japan). Melting points of the productswere determined on a BUCHIMelting Point Model B-540. A digital pH/mV/Ion meter (Metrohm 744, Switzerland) was used for preparing of the buffer solutions. All chemicals (solvents and phosphate salts, hydroquinone and thiouracil derivatives) were purchased from Merck (Darmstadt, Germany). These chemicals were used without further purification. 3. Results and discussion 3.1. Electrosynthesis of hydroquinonethioethers (3a–b)
In a typical reaction, a 100 mL of phosphate buffer solution (0.2mol L-1, pH 6) water/DMF (90/10), containing 0.5mmol of hydroquinone (1a) and 0.5mmol of 6-methyl-2-thiouracil (3a) or6- propyl-2-thiouracil (3b), was electrolyzed by five carbon electrodes in an undivided cell at 0.35 V vs. Ag/AgCl. The electrolysis was terminatedwhen the current decayed to 5% of its original value. The process was repeated several times during the electrolysis and the carbon electrodes were washed in acetone repeatedly reactivation. As hydroquinone was soluble in phosphate buffer solution (90 mL) and thiouracile derivatives (3a–3b) were already dissolved in a minimum amount of DMF (10 mL) so the mixture of them was homogeneous. Otherwise the obtained hydroquinonethioethers (6a–6b) would not be soluble under the mentioned conditions; therefore, at the end of the electrolysis, the obtained products (6a– 6b)wouldprecipitateout. Finally, eachof the formedprecipitatewas filtered and dried. After drying, purification of the product (6a–6b) was done by using of methanol/acetone (50/50). The resulting productwas characterizedbymeltingpoint, FT-IR, 1HNMR, 13CNMR and elemental analysis. 3.2. Characterization of products
2-(2,5-Dihydroxyphenylsulfanyl)-6-methyl-1H-pyrimidin-4- one (6a): Lightbrown solid; yield 82%; mp > 300 ℃ (decomposed); FT-IR (KBr, cm-1): 3240 (broad, OH aromatic), 1666 (C=O), NH peak covered by OH peak; 1H NMR (300 MHz, DMSO-d6): δ 1.96 (s, 3H, methyl), 5.90 (s, 1H, CH), 6.50–7.10 (m, 3H, aromatic), 9.90 (broad, 2H, OH, exchanged with D2O), 12.32 (s, 1H, NH, exchanged with D2O); 13C NMR (75 MHz, DMSO-d6): δ 18.5, 105.2, 114.1, 117.8, 121.1, 127.2, 143.9, 145.1, 160.2, 162.5, 164.44; Anal. Calcd. for C11H10N2O3S: C, 52.79; H, 4.03; N, 11.19; S, 12.81. Found: C, 52.91; H, 4.08; N, 11.02; S, 12.61.
2-(2,5-Dihydroxyphenylsulfanyl)-6-propyl-1H-pyrimidin-4- one (6b): Brown solid; yield 80%; mp > 300 ℃ (decomposed); FTIR (KBr, cm-1): 3200 (broad, OH aromatic), 1643 (C=O), NH peak covered by OH peak; 1H NMR (300 MHz, DMSO-d6): δ 0.86 (t, 3H, CH3), 1.35–1.55 (m, 2H, CH2), 2.2 (t, 2H, CH2), 5.89 (s, 1H, CH), 6.5– 7.05 (m, 3H, aromatic), 9.7 (broad, 2H, OH, exchanged with D2O), 12.35 (broad, 1H, NH, exchanged with D2O); 13C NMR (75 MHz, DMSO-d6): δ 13.4, 20.8, 38.2, 105.2, 108.2, 113.1, 121.1, 128, 144.9, 145.6, 160, 165.2, 168.6; Anal. Calcd. for C13H14N2O3S: C, 56.10; H, 5.07; N, 10.06; S, 11.52. Found: C, 56.25; H, 5.13; N, 9.92; S, 11.35. 3.3. Effect of pH value
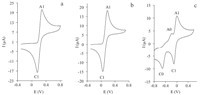
The pH value is one of the most important factors in the electrosynthesis of hydroquinonethioethers (6a–b). The optimization of pH values from 4 to 9 was done by using of H3PO4 (0.2 mol L-1) and NaOH (0.2 mol L-1) solutions. To attain accurate pH value, the reaction mixture was monitored by a pH-meter. The results indicated that the peak current ratio (ICl P =IAl P ) for hydroquinone (1a) (in the absence of 3a or 3b) decreases at higher pH values, because of some unwanted side reactions such as dimerization and hydroxylation reactions [10,11] on hydroquinone (Fig. 1). As can be seen in Fig. 1, under basic conditions (pH 9), the voltammogram exhibits two cathodic peaks (C1 and C0) and two anodic peaks (A0 and A1). These new peaks (C0 and A0) are related to the electrochemical behaviors of the side products. In contrast, under acidic conditions anion of thiouracil derivatives (4a–b) can be protonated and inactivated in the Michael addition reaction with p-quinone (2a). Therefore, we chose phosphate buffer solution (0.2 mol L-1, pH 6) as an optimal pH to achieve the maximum amount of pure product.
|
Download:
|
| Fig. 1.Cyclic voltammograms of 2 mmol L-1 hydroquinone (1a) in buffer solution/DMF (90/10, v/v) mixture with various pHs. The pHs are: (a) 4.0, (b) 6.0, (c) 9.0, scan rate: 50 mV s-1, T = 25 ± 1 ℃. | |
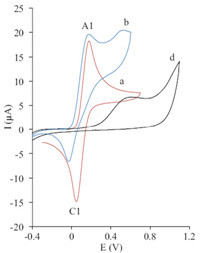
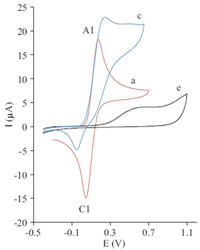
Cyclic voltammogram of a solution of 2 mmol L-1 of hydroquinone (1a) in water/DMF (90/10) containing phosphate buffer (0.2 mol L-1, pH 6) showed one anodic peak (A1) and the corresponding cathodic peak (C1), which demonstrated the transmutation of hydroquinone (1a) to p-quinone (2a) and vice versa within a quasi-reversible two-electron process (Figs. 2 and 3, curve a). A peak current ratio (ClP=IAlP) of nearly unity, particularly during the recycling of the potential, can be considered as a criterion for the stability of 2a produced at surface of the electrode under the experimental conditions [4,5]. The oxidation of 1a in the presence of 6-methyl-2-thiouracil (3a) and 6-propyl-2-thiouracil (3b) as nucleophiles were studied in details. Fig. 2(curve b) shows the cyclic voltammogram obtained for a 2 mmol L-1 solution of 1a in the presence of 2 mmol L-1 3a and Fig. 3(curve c) shows the cyclic voltammogram obtained for a 2 mmol L-1 solution of 1a in the presence of 2 mmol L-1 3b.
|
Download:
|
| Fig. 2.Cyclic voltammograms of 2 mmol L-1 hydroquinone (1a) in the absence (a), presence (b) of 2 mmol L-1 6-methyl-2-thiouracil (3a) and cyclic voltammograms of 2 mmol L-1 3a (d) at glassy carbon electrode in 0.2 mol L-1 phosphate buffer/ DMF (90/10, v/v), pH 6.0, scan rate: 50 mV s-1, T = 25 ± 1 ℃. | |
|
Download:
|
| Fig. 3.Cyclic voltammograms of 2 mmol L-1 hydroquinone (1a) in the absence (a), presence (c) of 2 mmol L-1 6-propyl-2-thiouracil (3b) and cyclic voltammograms of 2 mmol L-1 3b (e) at glassy carbon electrode in 0.2 mol L-1 phosphate buffer/DMF (90/10, v/v), pH 6.0, scan rate: 50 mV s-1, T = 25 ± 1 ℃. | |
The cyclic voltammograms exhibit one anodic peak at about 0.25 V vs. Ag/AgCl, and the height of the cathodic peak (C1) decreases. Voltammograms of 2 mmol L-1 1a in the presence of 2 mmol L-1 3a and 3b at different scan rates are shown in Fig. 4. The height of the cathodic peak (C1) increases proportionally to the augmentation of potential sweep rate (Fig. 4). A plot of the peak current ratio (IPC1/IPA1) versus scan rate for a mixture of 1a and (3a or 3b), confirms the reactivity of 2a toward (3a or 3b). The peak current ratio (ClP=IAlP) increased at higher scan rates (Fig. 5) [12]. To prove the mechanism, the peak current function for the A1 peak (IAlP/v1/2) versus scan rate was plotted (data not shown). The peak current function was decreased at lower scan rate and such behavior is indicative of an electron transfer followed by chemical reaction (EC) mechanism [13].

|
Download:
|
| Fig. 4.Typical voltammograms of 2 mmol L-1 hydroquinone (1a) in the presence of (a) 2 mmol L-1 3a and (b) 2 mmol L-1 3a at glassy carbon electrode in 0.2 mol L-1 phosphate buffer/DMF (90/10, v/v), pH 6.0 and various scan rates. Scan rates from up to down are: 250, 150, 100, 50 and 10 mV s-1, respectively, T = 25 ± 1 ℃. | |

|
Download:
|
| Fig. 5.Variation of peak current ratio (ClP=IAlP) vs. scan rate for 2 mmol L-1 1a in the presence of 2 mmol L-1 3a (curve i) and 2 mmol L-1 1a in the presence of 2 mmol L-1 3b (curve j), T = 25 ± 1 ℃. | |
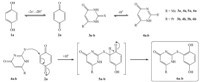
Controlled-potential coulometry was performed in a phosphate buffer solution (0.2 mol L-1, pH 6) and water/DMF (90/10) containing 2 mmol L-1 of 1a and 2 mmol L-1 of 3a or 3b at 0.35 V vs. Ag/AgCl. The monitoring of the progress of the coulometry was carried out by cyclic voltammetry (Fig. 6). It is shown that, proportional to the progression of coulometry, anodic peak A1 decreases and disappears when the charge consumption becomes about 2e- per molecule of 1a. These observations allowed us to propose the mechanism in Scheme 1 for the electrochemical oxidation of 1a in the presence of 3a and 3b. As indicated in Scheme 1, in phosphate buffer solution the thiouracile derivatives (3a–3b) could be deprotonated partially, like other similar compounds as indicated in literature [14], to produce the corresponding anions (4a–4b). These anions attack electrophiles by their sulfur atom rather than nitrogen atom because sulfur is a better nucleophile than nitrogen [15].

|
Download:
|
| Fig. 6.Cyclic voltammogram of 2 mmol L-1 hydroquinone in the presence of (a) 2 mmol L-1 3a and (b) 2 mmol L-1 3b at a glassy carbon electrode in 0.2 mol L-1 phosphate buffer/DMF (90/10, v/v), pH 6.0 (scan rate: 50 mV s-1, T = 25 ± 1 ℃) during controlled-potential coulometry at 0.35 V vs. Ag/AgCl. Progress of coulometry is associated with decreased anodic peak current. | |
|
Download:
|
| Scheme 1.Proposed mechanism. | |
The present work shows the nucleophilic addition of thiouracile derivatives to the p-quinone derived from electrochemical oxidation of hydroquinone in a buffer solution with pH 6, leading to the production of new hydroquinonethioethers. Results of voltammetric and spectrometric investigations revealed that thiouracile derivatives attacked to the p-quinone via its thiol moiety in a 1,4-Michael addition reaction and a new class of hydroquinonethioethers can be obtained. The final products (6a- 6b) can be obtained in relatively good yields and purity. Based on the coulometric, cyclic voltammetric studies and spectroscopic data, an EC mechanism is proposed for the electrochemical oxidation of hydroquinone in the presence of thiouracile derivatives.
AcknowledgmentsThe authors would like to thank from Semnan University Research Council for financial supports of this work.
| [1] | M.A. Ghanem, Electrocatalytic activity and simultaneous determination of catechol and hydroquinone at mesoporous platinum electrode, Electrochem. Commun. 9 (2007) 2501-2506. |
| [2] | N.Q. Ran, D.R. Knop, K.M. Draths, J.W. Frost, Benzene-free synthesis of hydroquinone, J. Am. Chem. Soc. 123 (2001) 10927-10934. |
| [3] | S.J. Li, Y. Xing, G.F.Wang,A graphene-based electrochemical sensor for sensitive and selective determination of hydroquinone, Microchim. Acta 176 (2012) 163-168. |
| [4] | S.S.H. Davarani, A.R. Fakhari, A. Shaabani, et al., A facile electrochemical method for the synthesis of phenazine derivatives via an ECECC pathway, Tetrahedron Lett. 49 (2008) 5622-5624. |
| [5] | D. Nematollahi, B. Dadpou, Electrochemical pyridination of hydroquinone in aqueous solution, Monatsh. Chem. 142 (2011) 1235-1239. |
| [6] | D. Nematollahi, A. Amani, E. Tammari, Electrosynthesis of symmetric and highly conjugated benzofuran via a unique ECECCC electrochemical mechanism: evidence for predominance of electrochemical oxidation versus intramolecular cyclization, J. Org. Chem. 72 (2007) 3646-3651. |
| [7] | A.R. Fakhari, H. Ahmara, S.S.H. Davarania, et al., Electro-organic synthesis of 2- amino-3-cyano-benzofuran derivatives using hydroquinonesandmalononitrile, Synth. Commun. 41 (2011) 561-568. |
| [8] | S.S.H. Davarania, D. Nematollahi, M. Shamsipur, et al., Electrochemical oxidation of 2,3-dimethybydroquinone in the presence of 1,3-dicarbonyl compounds, J. Org. Chem. 71 (2006) 2139-2142. |
| [9] | S. Shahrokhian, A. Hamzehloei, Electrochemical oxidation of catechol in the presence of 2-thiouracil: application to electro-organic synthesis? Electrochem. Commun. 5 (2003) 706-710. |
| [10] | D. Nematollahi, V. Hedayatfar, Diversity in electrochemical oxidation of dihydroxybenzenes in the presence of 1-methylindole, J. Chem. Sci. 123 (2011) 709- 717. |
| [11] | M.D. Ryan, A. Yueh, W.Y. Chen, The electrochemical oxidation of substituted catechols, J. Electrochem. Soc. 127 (1980) 1489-1495. |
| [12] | A.J. Bard, Electrochemical Methods, 2nd ed., Wiley, New York, 2001, pp. 103-497. |
| [13] | S.S.H. Davarani, F.N. Sheijooni, N.H. Arvin, F. Moradi, Electrochemical synthesis of 6-amino-5-(3,4-dihydroxyphenyl) pyrimidine, Tetrahedron Lett. 49 (2008) 710- 714. |
| [14] | S.S.H. Davarani, D. Nematollahi, M. Shamsipur, An efficient electrochemical method for synthesis of (1H-1,2,4-triazol-3-ylthio)benzen-1,2-diol derivatives, Heteroat. Chem. 18 (2007) 644-649. |
| [15] | L. Fotouhi, S. Taghavi, D. Nematollahi, M.M. Heravi, Study of the oxidation of some catechols in the presence of 4-amino-3-thio-1,2,4-triazole by digital simulation of cyclic voltammograms, Int. J. Chem. Kinet. 39 (2007) 340-345. |




