b Department of Chemistry, Faculty of Science, Gonbad Kavous University, P.O. Box 163, Gonbad, Iran;
c Chemistry & Chemical Engineering Research Center of Iran, Tehran, Iran
1. Introduction
Multicomponent reactions (MCRs) are defined by three or more reactants joining in a one-pot procedure to afford a single product [1, 2, 3, 4]. They are economically and environmentally useful because multi-step syntheses frequently produce a large amount of waste because the complex isolation actions often involve uncomfortable, toxic,and hazardous solvents after each step [5, 6, 7, 8]. MCRs are absolutely suited for combinatorial library synthesis and are increasing utilized in discovery of new drugs and agrochemicals [9]. They represent a useful tool toward the one-pot synthesis of diverse and complex compounds as well as small and drug-like heterocycles [10, 11]. Green chemistry holds significant potential not only for the reduction of byproducts,waste,and energy consumption but also in the expansion of new methodologies toward new materials,using existing technologies [12]. Medicinal and pharmaceutical chemistry possibly have the best chance to capitalize the green chemistry technologies [13]. For the perspective of the eco-friendly ‘‘green chemistry’’,a reaction should ideally,be conducted under solvent-free conditions with minimal or no side-product formation and with utmost atomeconomy [14]. Heterocycles are key compounds in the development of modern pharmaceutical chemistry,which is the reason why the design of amenable synthetic approaches for new heterocyclic systems is still significant challenge [15]. The thiazolium ring present in vitamin B1 serves as an electron sink and its coenzyme form is important for the decarboxylation of keto-acids [16]. Several pesticides possessing a heterocycle with an S or an N atom are known in agriculture. A large numbers of heterocycles have emerged as active pharmaceutical ingredients in several drugs for their potential anti-inflammatory [17, 18],anti-tumor [19] antihyperlipidemic [20],anti-hypertensive [21],anti-HIV infections [22],and several other biological properties [23, 24].
Hence,we investigated a simple three-component reaction between activated acetylenic compounds,primary amines and isothiocyanates in the presence of a catalytic amount of Nmethylimidazole under solvent-free conditions at room temperature which afforded 1,3-thiazolane derivatives 4 in good isolated yields. Propargylic esters gave lower yields (Scheme 1).
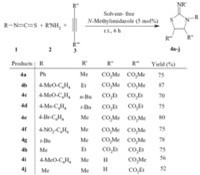
|
Download:
|
| Scheme 1.Synthesis of compound 4 using primary amines,activated acetylenic compounds and isothiocyanates. | |
All chemicals used in this work were purchased from Fluka (Buchs,Switzerland) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C,H,and N were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MAT 8430 spectrometer operating at an ionization potential of 70 eV. IR spectra were measured on a Shimadzu IR-460 spectrometer. 1HNMR and 13CNMR spectra were measured with a BRUKER DRX-500 AVANCE spectrometer at 500.1 and 125.8 MHz,respectively,using CDCl3 as a solvent and TMS as an internal standard or 85% H3PO4 as an external standard.
General procedure for preparation of compounds 4 and 12: To a magnetically stirred mixture of activated acetylenes 3 (2 mmol) and N-methylimidazole (5 mol%) was added a mixture of isothiocyanates 1 and primary amines 2 or secondary amine 11 (2 mmol) at room temperature. The reaction mixture was then stirred. After the completion of the reaction [TLC (AcOEt/hexane 1:7) monitoring],15 mL of H2O was poured into the reaction mixture. The solid residue was filtered and washed by cold diethyl ether to afforded pure compounds 4 and 12. 3. Results and discussion
The structures of compounds 4a-j were apparent from the 1HNMR, 13C NMR and IR spectra which are in agreement with the proposed structures. For example,the 1H NMR spectrum of 4a displayed two signals for vicinal methine protons at δ 4.78 and δ 4.92,which appeared as two doublets with 3JHH values of 12.4 Hz. The methoxy groups showed two singlets at δ 3.78 and δ 3.85. Observation of 3JHH = 12.4 Hz for the vicinal methine protons in 4a indicates the dominance of anti arrangement. Since compound 4 possesses two stereogenic centers,two enantiomers with anti HCCH arrangements are possible (Fig. 1). The carbonyl groups resonances in the 13C NMR spectra of 4a appeared at δ 172.5 (C=O),δ 173.7 (C=O). Also the mass spectra of 4a displayed the molecular ion peak with the correct m/z values.
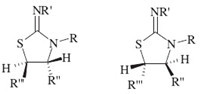
|
Download:
|
| Fig. 1.Anti arrangement of 4. | |
A proposed mechanism for the formation of compound 4 is shown in Scheme 2. Apparently,the zwitterionic intermediate 6 that formed from the reaction of N-methylimidazole (X3N) and the electron deficient acetylenic ester 3 is protonated by the intermediate 5 that was generated in situ from the reaction of primary amine 2 and isothiocyanate 1,producing intermediates 7 and 8. Nucleophilic attack of the conjugate base 7 on intermediate 8 leads to adduct 9,which undergoes a proton transfer process to afford a new zwitterion 10. Finally,intramolecular cyclization of 10 with the elimination of N-methylimidazole produces compound 4.

|
Download:
|
| Scheme 2.Proposed mechanism for the synthesis of compound 4. | |
Under similar conditions,these reactions proceed well with secondary amines but the yields of reactions are lower (Scheme 3).

|
Download:
|
| Scheme 3.Reaction of activated acetylenes,isothiocyanates and secondary amins in the presence of catalytic amount of N-methyl imidazole. | |
The configuration of compound 12b is confirmed by nuclear Overhauser effect (NOE) measurements. Thus,when the olefin signal was irradiated,the C55NCH3 protons were enhanced by about 10%,while the N(CH3)2 protons showed no significant enhancement. Thus,the configuration was deduced from the NOE measurements and the same configuration was assumed for the other derivatives of 12. 4. Conclusion
In conclusion,we found that the reaction of activated acetylenic compounds with isothiocyanates and primary amines in the presence of a catalytic amount of N-methylimidazole leads to a facile formation of some functionalized 1,3-thiazolanes under solvent-free conditions without using any additional catalyst. Also, butendioate and acrylate derivatives can be synthesized via the reaction of activated acetylenic compounds with isothiocyanate and secondary amines in the presence of a catalytic amount of Nmethylimidazole.
| [1] | A. Dömling, Isocyanide based multi component reactions in combinatorial chemistry, Comb. Chem. High Throughput Screening 1 (1998) 1-22. |
| [2] | A. Dömling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3169-3210. |
| [3] | L. Weber, Multi-component reactions and evolutionary chemistry, Drug Discov. Today 7 (2002) 143-147. |
| [4] | A. Shaabani, A. Maleki, A.H. Rezayan, A. Sarvary, Recent progress of isocyanidebased multicomponent reactions in Iran, Mol. Divers. 15 (2011) 41-68. |
| [5] | J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005. |
| [6] | P. Wipf, C. Kendall, Novel applications of alkenyl zirconocenes, Chem. Eur. J. 8 (2002) 1779-1784. |
| [7] | G. Balme, E. Bossharth, N. Monteiro, Pd-assisted multicomponent synthesis of heterocycles, Eur. J. Org. Chem. (2003) 4101-4111. |
| [8] | A. Jacobi von Wangelin, H. Neumann, D. Gordes, et al., Multicomponent coupling reactions of aldehydes and amides: an efficient tool for organic synthesis, Chem. Eur. J. 9 (2003) 4286-4294. |
| [9] | (a) A. Domling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3168-3210; (b) I. Ugi, A. Domling, Multicomponent reactions in organic chemistry, Endeavour 18 (1994) 115-122; (c) S. Heck, A. Domling, A versatile multi-component one-pot thiazole synthesis, Synlett (2000) 424-426. |
| [10] | L. Weber, The application of multi-component reactions in drug discovery, Curr. Med. Chem. 9 (2002) 2085-2093. |
| [11] | I. Yavari, A.S. Shahvelayati, A. Malekafzali, Efficient synthesis of functionalized 2 4-diaminothiazoles from tetramethylguanidine, isothiocyanates, and a-bromoketones, J. Sulfur Chem. 31 (2010) 499-508. |
| [12] | G.W.V. Cave, C.L. Raston, J.L. Scott, Recent advances in solventless synthesis with remarkable versatility, Chem. Commun. (2001) 2159-2169. |
| [13] | R.A. Sheldon, Catalysis: the key to waste minimization, Chem. Ind. (1997) 12-15. |
| [14] | G. Nagendrappa, Organic synthesis under solvent-free condition: an environmentally benign procedure, Resonance 7 (2002) 59-68. |
| [15] | P.M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, 2nd ed., Wiley, Chichester, UK, 2002. |
| [16] | R. Breslow, On the mechanism of thiamine action. IV. Evidence from studies on model systems, J. Am. Chem. Soc. 80 (1958) 3719-3726. |
| [17] | S. Miwatashi, Y. Arikawa, E. Kotani, et al., Novel Inhibitor of p38 MAP kinase as an anti-TNF-drug: discovery of N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5- yl]-2-pyridyl]benzamide (TAK-715) as a potent and orally active anti-rheumatoid arthritis agent, J. Med. Chem. 48 (2005) 5966-5979. |
| [18] | C. Papadopoulou, A. Geronikaki, D. Hadjipavlou-Litina, Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides derivatives, Il Farmaco 60 (2005) 969-973. |
| [19] | Y. Kumar, R. Green, K.Z. Borysko, et al., Synthesis of 2,4-disubstituted thiazoles and selenazoles as potential antitumor and antifilarial agents. 1. Methyl 4- (isothiocyanatomethyl)thiazole-2-carbamates-selenazole-2-carbamates, and related derivatives, J. Med. Chem. 36 (1993) 3843-3848. |
| [20] | R. Pereira, C. Gaudon, B. Iglesias, et al., Synthesis of the PPAR-selective agonist GW501516 and C4-thiazole-substituted analogs, Bioorg. Med. Chem. Lett. 16 (2006) 49-54. |
| [21] | Y. Tsurumi, H. Ueda, K. Hayashi, et al., WS75624 A and B, new endothelin converting enzyme inhibitors isolated from Saccharothrix sp. No. 75624, J. Antibiot. 48 (1995) 1066-1072. |
| [22] | F.W. Bell, A.S. Cantrell, M. Hoberg, et al., Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors 1. Synthesis and basic structure-activity relationship studies of PETT analogs, J. Med. Chem. 38 (1995) 4929-4936. |
| [23] | D.S. Millan, R.H. Prager, C. Brand, P.H. Hart, The synthesis and activity of oxazole and thiazole analogues of urocanic acid, Tetrahedron 56 (2000) 811-816. |
| [24] | W.L. Wang, D.Y. Yao, M. Gu, et al., Synthesis and biological evaluation of novel bisheterocycle-containing compounds as potential anti-influenza virus agents, Bioorg. Med. Chem. Lett. 15 (2005) 5284. |




