b Department of Analytical Chemistry, Faculty of Chemistry, University College of Science, University of Tehran, Tehran 14155-6455, Iran;
c Department of Chemistry, Payame Noor University, Tehran 19395-3697, Iran
1. Introduction
Transition metal composition of environmental samples is of interest because of their essential or toxic nature. The accurate and precise determination of these ions is one of the main challenges of analytical chemists. Several analytical techniques are available for the determination of trace metals with sufficient selectivity for most applications. Due to its relative low cost and good analytical performance,flame atomic absorption spectroscopy (FAAS) is the main instrument for the determination of metals in many laboratories. However,the determination of metals at low levels by FAAS is difficult due to the poor sensitivity of FAAS and the interfering effects of alkali and alkaline earth group elements in the determinations [1, 2]. In order to overcome these difficulties, preliminary separation and preconcentration steps are necessary to eliminate any matrix components and improve the detection limit. Solid phase extraction (SPE) is an extraction method that uses a solid phase and a liquid phase to isolate one or one type of analyte from a solution. SPE method had the advantages of being more sensitive and environment friendly,simpler,faster and sampler saving [3, 4, 5]. The most prominent supports used are silica gel,activated carbon,Amberlite XAD resins,naphthalene,nanomaterial, bamboo charcoal and layered double hydroxide [6, 7, 8, 9, 10, 11]. Due to the high surface area of graphene,it can be used as a good adsorbent for separating different kinds of materials. Graphene oxide (GO) has many carbonyl,carboxylic,alcoholic groups on its monolayer surface that can be used as functional groups [12].
The goal of the present work is to fabricate graphene oxide (GO) as a solid phase adsorbent. The possibility of using 2-(tert-butoxy)- N-(3-carbamothioylphenyl)acetamide (TCTA) as a chelating reagent of Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions on a column to determine trace metal ions by FAAS was investigated. 2. Experimental
The instrumental detection system used was a Shimadzu model AA-630 atomic absorption spectrometer with a deuterium lamp background correction. A Metrohm 691 pH/ion-meter with a combined glass-calomel electrode was employed for measuring pH values. A glass column of 100 mm in length with an inner diameter of 8 mm,equipped with porous frits,was filled up to a height of about 10 mm with a suspension of GO (30.0 mg). Prior to use,the column was preconditioned with methanol (10.0 mL) and deionized water (10.0 mL),respectively. For conditioning of the column to a desired pH value,a phosphate buffer solution (5.0 mL, 0.2 mol/L) was used. After the elution,the column was washed with the eluting solution (10-15 mL) and water,subsequently. The size and morphology of GO were observed by transmission electron microscopy (TEM) using a CM120 microscope (Philips, Netherlands). Raman spectroscopy was carried out using a SENTERRA dispersive Raman microscope (BRUKER,Germany). Fourier transform-infrared (FT-IR) spectra were taken on a BRUKER VECTOR 22. The flow of the sample and eluent through the column was adjusted using a 10 roller peristaltic pump (Ultrateck Labs Co., Iran). Inorganic chemicals were of analytical grade and were purchased from Merck (Germany).
TCTA was gifted from Tehran University. GO was synthesized by the improved Hummers’ method [13]. In this method,graphite powder (1 g,Merck) was reacted with KMnO4 (6 g) in a mixture of concentrated H2SO4/concentrated H3PO4 (9:1,50 mL) solution. Synthesized GO was characterized by TEM,Raman and FT-IR spectroscopy.
Preconcentration of the metal ions of interestwas investigated from model solutions prior to the determination in the real samples. The desired mass of GO (30.0 mg) was loaded into the column and preconditioned as described. The pH value of the sample solution (100 mL,containing 10.0 μg/L ofmetal ions) was adjusted to 6.0 using a solution of phosphate buffer and a TCTA solution (50 μL,1.0 × 10-2 mol/L) was added. The sample solution (total 100 mL) was passed through the column after adjusting to the optimumpHvalue and the flowrate. The stripping of the metal ions from the GO column was achieved by eluting with HNO3 solution (3.0 mol/L) and the amount of desired ions in the eluent was determined by FAAS. The proposed method was applied to water,soil,blood and tomato samples. The analyte concentrations in these samples were determined by FAAS and sampleswere prepared as according to the procedures reported in literature [14, 15, 16]. 3. Results and discussion
To understand the nature and structure of the desired complex between metal ions and TCTA,a fixed amount of Fe(Ⅲ),Ni(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) ions were extracted into acetonitrile with various amounts of TCTA. The spectrophotometric studies revealed that TCTA as a chelating agent can form a fairly stable complex with metal ions with a 1:1 stoichiometry [17]. According to the results, quantitative recoveries (>95%) for Cu(Ⅱ) and Zn(Ⅱ) were obtained in a pH range of 5-7. In the pH range of 5-6.5,quantitative recoveries were obtained for Fe(Ⅲ). Also,Ni(Ⅱ) ions were quantitatively recovered at pH 6. However,metal ions form complexes and precipitates with OH- and their retention is changed and the recovery of the method is decreased at higher pH values. The slight decrease of the uptake in the acidic media may be attributed to the protonation of the lone pair of the nitrogen and sulfur that hinder the complex formation,due to competition of the protons with the metal ions for binding to TCTA. Therefore,the subsequent experiments were conducted at pH 6.0.
The proposed method is based on the sorption of the metal ions-TCTA chelates on GO. In the absence of TCTA,the analytes ions were not quantitatively recovered. With the addition of TCTA up to 4.0 mL (1 × 10-2 mol/L),the recovery values of the analyte ions increased. This value of TCTA is sufficient for the separation/ preconcentration procedure because of the very low level of the metal ion concentrations in real samples. With higher volume of the ligand,the recoveries were below 95% due to the competition in adsorption between the TCTA-metal chelates and the excess TCTA in the solution. Based on this,for all ensuing experiments, 3.0 mL of TCTA (1 × 10-2 mol/L) was chosen to account for other extractable species that might interfere with the assaying of metal ions.
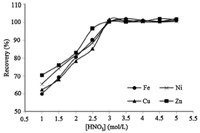
The effect of different solutions on the elution of the analyte ions of interest on GO was examined under partially optimized conditions. As can be seen in Fig. 1,the analyte ions sorbed quantitatively on the GO could be eluted with HNO3 (3.0 mol/L). In contrast to several previous reports,we found it was not necessary to extract the analyte ions with HNO3 in acetone. The eluent was evaporated to near dryness at 35 ℃ and diluted with HNO3 before the determination by FAAS.
|
Download:
|
| Fig. 1.Effect of nitric acid concentration on the recoveries of analyte ions. | |
The influence of the sample flow rate was investigated by passing sample solutions (50 mL,1.0 μg/L of studied cations) through the column flow rates in the range of 1-15 mL/min (other conditions were kept constant). The results showed that the extraction is independent of loading flow rates in the range of 1- 9 mL/min. When the flow rate was greater than 9 mL/min,there was a decrease in the recovery of metal ions. The reason for this decrease is probably the insufficient contact between the metal ions and the sorbent that did not allow the system to reach an equilibrium. This flow rate (9 mL/min) was therefore selected as the sample flow rate for further experiments. The effect of elution flow rate on recovery of analytes was investigated by keeping the elution volume at 5 mL of HNO3 (3.0 mol/L). The results showed that when the elution flow rates varied in the range of 1-3.5 mL/ min,the analytes can be recovered quantitatively. Therefore,the rate of flow for the eluent solution was maintained at 3.5 mL/min in all subsequent experiments.
Due to the low concentrations of trace metals in real samples (using samples with large volumes),a concentrating step was necessary [15]. The break-through volume of the sample solution was tested by dissolving metal ions (each containing 1.0 μg) in various volumes of water (100-2000 mL) and the recommended procedure under optimal experimental conditions was followed. The recoveries of the analyte ions were quantitative up to 1250 mL. Consequently,considering the final elution volume of 5 mL and optimum volume of 1250 mL,the preconcentration factors were 250 for the analytes.
In order to evaluate the possibility of selective recovery of analyte ions in the presence of Na+,K+,Mg2+,Ca2+,Al3+,Mn2+ and Co2+ and major anions of the real samples (nitrate,chloride and sulphate ions),the procedure has been performed with solutions containing such ions. A multi-element model solution (100 mL) containing 15.0 μg of Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ),Zn(Ⅱ) ions and individual and/or mixed foreign ions at various concentrations was prepared. The recovery of metal ions was investigated with the optimized procedure. The results showed that in the presence of 50-20000-fold electrolytes,the recovery of analyteswas above 95%. These results indicate that the present GO-based SPE can be suitably used as sorbents for Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions in concentrated electrolytes.
To determine the loading capacity of the GO column,sample solutions (100.0 mL) containing different Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions concentrations were adjusted to pH 6.0 with a phosphate buffer and the proposed procedure described was applied. The breakthrough curves for metal ions were obtained by plotting the concentration versus the mass of Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions adsorbed (in mg) per gram of GO. The retention capacity of GO for Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions was 6.7,6.4, 6.0 and 6.1 mg/g,respectively.
The effect of sample processing volume on analyte recovery was studied and the results show that the responses increased linearly with volume. For a sample solution,the calibration graph exhibits linearity over the range of 0.02-2.50,0.04-2.70,0.01-2.50 and 0.01-2.20 μg/mL with a correlation of 0.9999,0.9999,0.9998 and 0.9998 for Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ion,respectively.
The limit of detection (LOD) of the presented SPE study was established under the optimal experimental conditions after application of the preconcentration procedure to blank solutions. Typically,the limit of detection is three times the standard deviation of the blank and was found to be 0.39,0.11,0.63 and 0.45 ng/mL for Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ion,respectively. The analytical procedure was validated by determining the certified reference materials TMDA-54.4 fortified lake water with a certified content of iron,TMDW-500 drinking water with a certified content of nickel and copper,SLEW-3 with a certified content of zinc and SLRS-4 river water with a certified content of all ions mentioned above. As shown in Table 1,the results revealed a remarkable agreement between the observed values and certified values.
| Table 1 The levels of analyte ions in certified reference materials after application of the presented SPE method. |
The presented method was then applied for the determination of Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions in different samples,including water,blood,tomato,spinach and soil samples. The recoveries were in the range of 98.5%-105.0%,which demonstrates that this method is operative well for SPE of the ions studied in these matrices. 4. Conclusion
In the presented study,a simple,low cost and environmentally friendly SPE technique was developed based on the preconcentration of environmental samples containing Fe(Ⅲ),Ni(Ⅱ),Cu(Ⅱ) and Zn(Ⅱ) ions. This technique is based on the sorption of metal ions- TCTA chelates on synthesized GO prior to the determination by FAAS. The analytes can be selectively extracted from solutions at pH 6.0 even in the presence of alkali,alkaline earth,transition and heavy metal ions. Low detection limits and a high preconcentration factor of 250 are the main advantages of this procedure used in environmental ultra trace analyses. The developed method can be successfully applied in the determination of low level metal ions in water,blood,soil,tomato,and spinach samples with good results.
| [1] | M.R. Ganjali, M.R. Pourjavid, L. Haji-agha Babaei, et al., Ultra-trace monitoring of copper in environmental and biological samples by inductively coupled plasma atomic emission spectrometry after separation and preconcentration by using octadecyl silica membrane disks modified by a new Schiff's base, Quim. Nova 27 (2004) 213-217. |
| [2] | J.P. Xiao, Q.X. Zhou, H.H. Bai, Preconcentration of copper with multi-walled carbon nanotubes pretreated by potassium permanganate cartridge for solid phase extraction prior to flame atomic absorption spectrometry, Chin. Chem. Lett. 18 (2007) 714-717. |
| [3] | J.J. Ren, H.Y. Liu, Y.H. Hao, et al., Determination of resveratrol in red wine by solid phase extraction-flow injection chemiluminescence method, Chin. Chem. Lett. 18 (2007) 985-988. |
| [4] | M.R. Ganjali, M.R. Pourjavid, L.H. Babaei, et al., Octadecyl silica membrane disks modified with a new schiff's base for the preconcentration of lead and copper before their determination in water samples, Ann. Chim. Rome 94 (2004) 447- 456. |
| [5] | H.W. Zhang, K. Li, Z.X. Liang, et al., Development of a monolithic polymer pipette for solid-phase extraction of liquiritigenin in rat plasma, Chin. Chem. Lett. 23 (2012) 723-726. |
| [6] | X.N. Zhao, Q.Z. Shi, G.H. Xie, et al., TiO2 nanotubes: a novel solid phase extraction adsorbent for the sensitive determination of nickel in environmental water samples, Chin. Chem. Lett. 19 (2008) 865-867. |
| [7] | Y.Y. Jiang, Y.W. Wu, J.F. Liu, et al., Ammonium pyrrolidine dithiocarbamatemodified activated carbon micro-column extraction for the determination of As(Ⅲ) in water by graphite furnace atomic absorption spectrometry, Microchim. Acta 161 (2008) 137-142. |
| [8] | X.F. Su, X.N. Zhao, G.H. Xie, et al., Fluorometric determination of nonylphenol in water samples enriched with zirconium doped titanium dioxide nanotubes solid phase extraction, Chin. Chem. Lett. 23 (2012) 969-972. |
| [9] | Z.H. Wang, J.F. Xia, Q. Han, et al., Multi-walled carbon nanotube as a solid phase extraction adsorbent for analysis of indole-3-butyric acid and 1-naphthylacetic acid in plant samples, Chin. Chem. Lett. 24 (2013) 588-592. |
| [10] | R.S. Zhao, Y.L. Liu, J.B. Zhou, et al., Bamboo charcoal as a novel solid-phase microextraction coating material for enrichment and determination of eleven phthalate esters in environmental water samples, Anal. Bioanal. Chem. 405 (2013) 4993-4996. |
| [11] | Y.L. Liu, J.B. Zhou, R.S. Zhao, et al., Using Zn/Al layered double hydroxide as a novel solid phase extraction adsorbent to determine polycyclic aromatic hydrocarbons at trace levels in water samples by gas chromatography-mass spectrometry, Anal. Bioanal. Chem. 404 (2012) 1603-1610. |
| [12] | Y.W. Zhu, S. Murali, W.W. Cai, et al., Graphene and graphene oxides: synthesis, properties, and applications, Adv. Mater. 22 (2010) 3906-3924. |
| [13] | D.C. Marcano, D.V. Kosynkin, J.M. Berlin, et al., Improved synthesis of graphene oxide, ACS Nano 4 (2010) 4806-4814. |
| [14] | M. Ghaedi, K. Niknam, A. Shokrollahi, et al., Flame atomic absorption spectrometric determination of trace amounts of heavy metal ions after solid phase extraction using modified sodium dodecyl sulfate coated on alumina, J. Hazard. Mater. 155 (2008) 121-127. |
| [15] | F. Marahel, M. Ghaedi, M. Montazerozohori, et al., Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples, Food Chem. Toxicol. 49 (2011) 208-214. |
| [16] | M. Ezoddin, F. Shemirani, K. Abdi, et al., Application of modified nano-alumina as a solid phase extraction sorbent for the preconcentration of Cd and Pb in water and herbal samples prior to flame atomic absorption spectrometry determination, J. Hazard. Mater. 178 (2010) 900-905. |
| [17] | M.R. Pourjavid, A. Akbari Sehat, Preparation of a modified octadecyl silica membrane disk for solid phase extraction of lead(Ⅱ) ions in different real samples and their determination with ICP-AES, J. Membr. Sep. Technol. 1 (2012) 9-18. |