b Beijing Institute of Pharmacology and Toxicology, Beijing 100850, China
1. Introduction
Nitric oxide (NO) mediates multiple physiological and pathological processes [1]. Previous studies have demonstrated that NO can protect the liver from injury and ameliorate the progress of liver fibrosis or cirrhosis. NO-releasing drugs may represent an important therapy for liver damage,fibrosis,and cirrhosis [2, 3]. However,non-specific NO-releasing drugs such as isosorbide-5- mononitrate induce significant reduction of arterial pressure, which limits their clinical application [4].
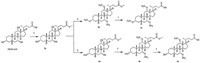
Liver targeted therapy can deliver chemotherapeutic drugs selectively to the liver to avoid side effects or toxicities. Bile acids are the only small molecules with high oral availability that are taken in specifically by the liver [5, 6]. Conjugating NO releasing components with bile acids such as cholic acid (CA) or ursodeoxycholic acid (UDCA) can deliver NO selectively to the liver for the treatment of liver diseases without significant reduction of arterial pressure. NCX-1000 (Fig. 1) is the first liver-specific NO donor drug that can protect mice against acute liver injury induced by acetaminophen (APAP) or Con A [7, 8]. However,NCX-1000 has not met the endpoints in phase 2 clinical trials.

|
Download:
|
| Fig. 1.Structure of NCX1000 and targeted compounds. | |
NCX-1000 was prepared by conjugating the NO releasing moiety with the 24-COOH of UDCA by two ester bonds. However, the intact 24-COOH of bile acid is essential for liver specificity [5]. Conjugating with the 24-COOH may abolish the targeting of UDCA, and the ester bond in the conjugate is not stable in human blood.
Novel liver-specific nitric oxide (NO) releasing drugs were designed and synthesized by use of the hydroxyl group on the steroid nucleus at position 3,7,or 12 as the conjugating group with the preserved 24-COOH for hepatocyte specific recognition. Compounds 1a-1d were nitrates of 3-OH,7-OH,or 12-OH. In these compounds,the bile acids are used as both the NO carrier and targeting ligand.Compounds 1e and 1f werenitrates of 3-O(CH2)4OH and 3-O(CH2)2OH derivatives of UDCA,respectively. The linkage chain between nitrate and UDCA canmodulate the release rate ofNO from the conjugates.
Preliminary biological evaluation revealed that the targeted conjugates can protect mice against acute liver injury induced by APAP or carbon tetrachloride (CCl4). Both the site and number of nitration could affect the biological activity. Compounds 1e and 1f are two most active compounds and show more potent protective effects than NCX-1000. Further in vivo nitrate distribution investigation revealed that the nitrate level in the liver significantly increased after oral administration of 1e,while nitrate level in the blood did not significantly changed. Co-administration of UDCA significantly antagonized the increase of nitrate in the liver resulted by 1e,which demonstrated that the delivery of 1e to the liver was mediated via the bile acid transport system. 2. Experimental 2.1. Chemistry
The synthesis of compounds 1a and 1b is outlined in Scheme 1. The 24-COOH of bile acids was first protected by methylation in HCl-methanol solution to give 2a . Target compound 1a was obtained by nitration of all three hydroxyl groups of 2a with fuming nitric acid in Ac2O and then deprotection with 5% KOH menthol solution. The 3-OH of 2a was selectively reacted with acetyl chloride to give the 3-OH protected derivative 3b . Compound 1b was obtained by nitration of 3b followed by deprotection of 4b,similar to 1a.
|
Download:
|
| Scheme 1.Synthesis route of compounds 1a–1b. (a) HCl–methanol, r.t., 12 h; (b, e) acetic anhydride, fuming nitric acid, -5 ℃, 1 h; (c, f) 5% KOH menthol solution, reflux, 1 h; (d) acetyl chloride, pyridine, 0 ℃, 1 h, then r.t., 2 h. | |
Compounds 1c and 1d were synthesized as depicted in Scheme 2. The methylated derivatives ( 2a and 2d) of bile acids were reacted with Ac2O to give the acetate derivatives 3c and 3d; 3c and 3d were selectively deprotected to give the 3-OH free derivative 4c and 4d . The target compounds 1c and 1d were obtained by nitration and then deprotection as that of 1a.

|
Download:
|
| Scheme 2.Synthesis route of compounds 1c–1d. (a) acetic anhydride, pyridine, DMAP, r.t., 6 h; (b) HCl–methanol, r.t., 1–3 h; (c) acetic anhydride, fuming nitric acid, -5 ℃, 1 h; (d) 5% KOH menthol solution, reflux, 1 h. | |
Compounds 1e and 1f were prepared as outlined in Scheme 3. 4d was reacted with methanesulfonyl chloride to obtain the methanesulfonate derivative 6. Compound 6 was then reacted with 1,4-butanediol or ethylene glycol to give the hydroxyl alkylated derivatives 7e and 7f,respectively. Compounds 7e and 7f were nitrated and then deprotected to afford target compounds 1e and 1f,respectively. NCX-1000 was synthesized according to the literature [9].

|
Download:
|
| Scheme 3.Synthesis route of compounds 1e–1f. (a) methanesulfonyl chloride, pyridine, 0 ℃, 1 h, then r.t.,2 h; (b) butanediol or ethylene glycol, pyridine, 100 ℃, 2 h; (c) acetic anhydride, fuming nitric acid, -5 ℃, 1 h; (d) 5% KOH menthol solution, reflux, 1 h. | |
The structures of compounds 1a-1f were characterized [10]. 2.2. Pharmacology 2.2.1. Protective effect against acute liver injury induced by CCl4 or APAP
Baclb/c mice were randomly divided (n = 10,half male and half female). Compounds were suspended in 1% sodium methoxycellulose and administered by intragastric at dosage of 50 mg/kg. Sixty minutes after administration of the test compounds,0.1% CCl4 in olive oil at dose of 10 mL/kg or APAP at dose of 200 mg/kg was administrated by i.p. injection to induce the acute liver injury model. Saline was subcutaneous injected as normal control. Twelve hours after administration of carbon tetrachloride or APAP,same amount of the compounds was administered by intragastric. Twenty-four hours after the second administration of compounds,whole blood samples were taken from the suborbital sinus and the samples were centrifuged,the plasma was frozen and stored immediately at -70 ℃. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was determined with the Olympus AU5400 automated biochemistry analyzer (Olympus Corporation,Tokyo,Japan). 2.2.2. NO distribution in vivo
After overnight fasting,male Baclb/c mice were randomly divided into 4 groups (n = 10). Compounds were suspended in 1% sodium methoxycellulose and administered by intragastric injection. The mice in the first group were treated with saline as control; the mice in the second group were treated with 1e (10 mg/mL, 20 mL/kg); in the third group,mice were treated with UDCA (50 mg/mL,20 mL/kg); in the fourth group,mice were treated with UDCA (50 mg/mL,20 mL/kg) and 1e (10 mg/mL,20 mL/kg). Five mice from each group were sacrificed in 2 h or 4 h after administration of the compounds. Whole blood samples were taken from the suborbital sinus,and the samples were centrifuged. The plasma was frozen and stored immediately at -70 ℃. The livers were collected at the same time and rapidly frozen in liquid nitrogen and maintained at -80 ℃ until analysis. Liver lysates were prepared by homogenization in Tris-HCl buffer (0.01 mol/ L + NaCl 0.1 mol/L). The homogenates were centrifuged at 15,000 rpm for 15 min and the supernatant was taken for nitrate determination.
The nitrate concentration was measured by Griess assay using a colorimetric assay kit (Beyotime,China) [11]. Briefly,50 mL of the sample solutions were mixed with 50 μL Griess reagent Ⅰ and 50 μL Griess reagent Ⅱ,and the mixture was then placed at room temperature for 10 min. The absorbance was measured at 540 nm, and sodium nitrate solutions at different concentrations were used as positive controls for the standard curve.
All results are expressed as mean ± SD. For multiple comparisons statistical analysis was performed by one-way ANOVA followed by Tukey multiple comparison tests with SPSS 20.0 software. P < 0.05 was considered to be statistically significant. 3. Results and discussion 3.1. Protective effect against acute liver injury induced by CCl4 or APAP
Both CCl4 and APAP treatment resulted in significant increase of ALT and AST in the blood. As shown in Table 1,at doses of 50 mg/kg, compounds 1b,1d,1e,1f and NCX-1000 significantly inhibited the rise of both plasma ALT and AST in both the CCl4 and APAP models; compound 1a and UDCA showed some effects on both plasma ALT and AST in the CCl4 model but only plasma ALT in the APAP model; compound 1c significantly inhibited the rise of plasma AST in the CCl4 model and plasma ALT in the APAP model; CA has no effect on either plasma ALT or AST in the CCl4 or the APAP model. All the active compounds except 1c showed greater inhibitory effects on plasma ALT than on plasma AST.
| Table 1 Protective activity against CCl4 and APAP induced acute liver-injury.* |
Compounds 1e and 1f exhibited much more profound protective effects against both CCl4 and APAP induced hepatocyte injury than NCX-1000,UDCA,and 1a-1d. The difference in the activity between 1e or 1f and other target compounds may result from the difference in NO releasing rate from these compounds. Nitrate of the axial hydroxyl group on the steroid nucleus may not be easily reached by cytochrome P450 or glutathione-S-transferase, enzymes that are responsible for the hydrolysis of organic nitrate. In vitro NO releasing experiments revealed that 1a-1d release NO much more slowly than 1e or 1f after incubation with HepG2 cells (Fig. 2). Ursodeoxycholic acid is a hepatocyte protective agent [12]. The nitrates of ursodeoxycholic acid (1d-1f) showed better hepatocyte protective effects than the nitrates of cholic acid (1a-1c). This may be explained by the synergistic effect of UDCA and NO released from the target compounds 1d-1f.
|
Download:
|
| Fig. 2.NO release assay in vitro of target compounds. HepG2 cells were incubated in triplicate with each compound. The levels of nitrate produced in the lysates of HepG2 cells were determined using a calorimetric Griess assay [11]. | |
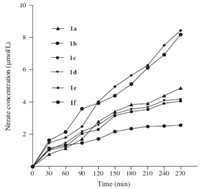
After absorption and transportation to the liver via the bile acid mediated route,the liver specific NO releasing compounds will release NO by cytochrome P450 or glutathione-S-transferase catalyzed hydrolysis,and the NO released is then transformed into nitrate. So the concentration of nitrate in liver and in blood was determined to evaluate the specificity of the target compound.
As shown in Fig. 3,the nitrate level in the liver is significantly increased at 2 h and 4 h after administration of 1e,while the change of nitrate level in the blood is not significant. UDCA has no effect on the nitrate levels in the liver. Co-administration of UDCA significantly antagonized the increase of nitrate in the liver resulted by 1e. These results indicated that the NO-releasing compounds we synthesized were delivered into the liver via the bile acid mediated transportation system route and released NO specifically in the liver.

|
Download:
|
| Fig. 3.Nitrate concentration in blood and liver after oral administration of 1e. | |
In conclusion,six novel liver specific NO releasing compoundswith bile acid as both the NO carrier and targeting ligand were designed and synthesized. Compounds 1a-1d were synthesized by selective nitration of the 3-OH,7-OH,or 12- OH on the steroid nucleus,while 1e and 1f were nitrates of 3- O(CH2)4 OH and 3-O(CH2)2OH derivatives of ursodeoxycholic acid. The intact 24-COOH is preserved in all the targeted compounds for hepatocyte specific recognition. All compounds showed protective effects against both CCl4 and APAP induced acute liver injury in mice models. Compounds 1e and 1f exhibited much more profound protective effects against both CCl4 and APAP induced hepatocyte injury than NCX-1000,UDCA, and 1a-1d. The nitrate level in the liver is significantly increased after administration of 1e while the change of nitrate level in the blood is not significant. Co-administration of UDCA significantly antagonized the increase of nitrate in the liver resulted by administration of 1e,indicating that the NO-releasing compounds were delivered specifically into the liver by the bile acid mediated transportation system.
AcknowledgmentsThis work was supported by the National High Technology Research and Development (863) Project (No. 2006AA02A4C6) and National Natural Science Foundation of China (Nos. 30572220 and 30972626).
| [1] | D.C. Rockey, V. Shah, Nitric oxide biology and the liver: report of an AASLD research workshop, Hepatology 39 (2004) 250-257. |
| [2] | M. Abu-Amara, S.Y. Yang, A. Seifalian, B. Davidson, B. Fuller, The nitric oxide pathway-evidence and mechanisms for protection against liver ischaemia reperfusion injury, Liver. Int. 32 (2012) 531-543. |
| [3] | N.M. Atucha, F.J. Nadal, D. Iyu, et al., Role of vascular nitric oxide in experimental liver cirrhosis, Curr. Vasc. Pharmacol. 3 (2005) 81-85. |
| [4] | L. Bellis, A. Berzigotti, J.G. Abraldes, et al., Low doses of isosorbide mononitrate attenuate the postprandial increase in portal pressure in patients with cirrhosis, Hepatology 37 (2003) 378-384. |
| [5] | A. Enhsen, W. Kramer, G. Wess, Bile acids in drug discovery, Drug Discov. Today 3 (1998) 409-418. |
| [6] | J. Li, L. Hai, W.J. Liu, X.C. Wu, Y. Wu, Study on synthesis and distribution in vivo of 5-Fu-cholic acid conjugate, Chin. Chem. Lett. 20 (2009) 136-138. |
| [7] | S. Fiorucci, E. Antonelli, E. Distrutti, et al., Liver delivery of NO by NCX-1000 protects against acute liver failure and mitochondrial dysfunction induced by APAP in mice, Br. J. Pharmacol. 143 (2004) 33-42. |
| [8] | S. Fiorucci, A. Mencarelli, B. Palazzetti, et al., An NO derivative of ursodeoxycholic acid protects against Fas-mediated liver injury by inhibiting caspase activity, Proc. Natl. Acad. Sci. U.S.A. 98 (2001) 2652-2657. |
| [9] | D.P. Soldato, S.A. Nicox, Pharmaceutical compounds, Patent WO 0061604, 2000- 10-19. |
| [10] | Data for new compounds. 1a: Yield 73%. Mp: 221-223 ℃; 1H NMR (400 MHz, DMSO-d6): δ 11.93 (s, 1H), 5.29 (s, 1H) 5.07 (s, 1H), 4.79 (s, 1H), 2.39-2.37 (m, 1H), 2.26-2.23 (m, 1H), 2.23-1.02 (m, 28 H), 0.81 (s, 3H). MS (FAB): m/z 542.6 (M-1). 1b: Yield 76%. Mp: 191-193 ℃; 1H NMR (400 MHz, DMSO-d6): δ 11.98 (s, 1H), 5.39 (s, 1H), 5.09 (s, 1H), 3.24 (s, 1H), 1.98-0.97 (m, 23H), 0.99-0.78 (m, 11H). MS (FAB): m/z 497.7 (M-1). 1c: Yield 67%. Mp: 218-220 ℃; 1H NMR (400 MHz, DMSO-d6): δ 11.93 (s, 1H), 4.85 (s, 1H), 4.20-4.18 (d, 2H), 3.79 (s, 1H), 3.63 (s, 1H), 1.99-0.97 (m, 30H), 0.59 (s, 3H). MS (FAB): m/z 452.5 (M-1). 1d: Yield 62%. Mp: 186-188 ℃; 1H NMR (400 MHz, DMSO-d6): δ 11.84 (s, 1H), 4.85 (s, 1H), 3.60- 3.57 (d, 2H), 2.24 (s, 1H), 2.02-0.97 (m, 25H), 0.94 (d, 3H, J = 6.5 Hz), 0.85 (s, 3H), 0.63 (s, 3H). MS (FAB): m/z 436.4 (M-1). 1e: Yield 47%. Mp: 185-187 ℃; 1H NMR (400 MHz, CDCl3): δ 11.96 (s, 1H), 4.56-4.52 (t, 2H), 3.86-3.84 (d, 1H, J = 7.0 Hz), 3.48 (s, 1H), 3.34-3.32 (t, 2H), 2.25-0.87 (m, 37H), 0.62 (s, 3H). MS (FAB): m/z 508.8 (M-1). 1f: Yield 43%. Mp: 174-176 ℃; 1H NMR (400 MHz, CDCl3): δ 12.05 (s, 1H), 4.56-4.54 (t, 2H), 3.90-3.87 (d, 1H, J = 6.8 Hz), 3.46 (s, 1H), 3.31-3.28 (t, 2H), 2.26-1.04 (m, 27H), 0.97 (s, 3H), 0.89 (d, 3H, J = 6.4 Hz), 0.61 (s, 3H). MS (FAB): m/z 480.5 (M-1). |
| [11] | M.D. Lu, X. Zhou, Y.J. Yu, et al., Synthesis and in vitro biological evaluation of nitric oxide-releasing derivatives of hydroxylcinnamic acids as anti-tumor agents, Chin. Chem. Lett. 24 (2013) 415-418. |
| [12] | Z. Xiang, Y.P. Chen, K.F. Ma, et al., The role of Ursodeoxycholic acid in nonalcoholic steatohepatitis: a systematic review, BMC Gastroenterol. 13 (2013) 140. |





