1. Introduction
In the last two decades,ionic liquids (ILs) have emerged as potential ‘greener’ alternatives to volatile organic solvents,and they have been used as environmentally friendly media for many important organic reactions [1]. A number of ionic liquids have been developed and used as catalysts for different reactions. Some acidic ionic liquids have been utilized to catalyze Friedel Crafts acylation [2],Knoevenagel condensation [3],esterification reaction [4],and cleavages of ether [5]. Basic ionic liquids have aroused unprecedented interest because they show more advantages,such as catalytic efficiency and recycling of the ionic liquid,which cannot be provided by the combination of inorganic base and ionic liquid for base catalyzed processes [6].
The Michael addition is one of the most important carbon- carbon bond forming reactions and is widely applied in organic syntheses [7]. This reaction is usually performed in organic solvent and is catalyzed by strong bases and Lewis acids that lead to undesirable side reactions [8]. Many catalysts have been developed to solve the above problems [9]. However,these catalysts have some limitations,such as long reaction time [9a, b, d, f, h],high reaction temperature [9c, f],use of large amount of catalysts [9g, 10],and low yields [9b, c, e, f, h]. A task specific ionic liquid, [Bmim]OH,was applied as a catalyst and reaction medium in Michael addition of active methylene compounds to conjugated ketones,carboxylic esters,and nitriles [1]. However,a large amount of catalyst was necessary to obtain high yield. Recently, another basic ionic liquid,[Bmim]OAc,was also used to catalyze the Michael addition of active methylene to α,β-unsaturated carboxylic esters and nitriles [11]. However,in this protocol, volatile and toxic organic solvents were used in order to achieve high yields. Recently,Xiaoming Feng et al. used a chiral organocatalyst in the Michael reaction [12]. In this letter,we report dabco-based basic ionic liquids (Fig. 1) as highly efficient and environmentally friendly catalysts for Michael addition of active methylene compounds to α,β-unsaturated carboxylic esters and nitriles at room temperature under solvent free conditions, providing excellent yields. The catalysts can be recycled many times without much loss of activity.

|
Download:
|
| Fig. 1.Structure of dabco-based ionic liquids. | |
All the reagents were commercial reagents of A.R. grade and were used without further purification. All the commercial solvents were used after distillation. Melting points were determined by using a Veego melting point apparatus-I and were uncorrected. IR spectra were taken using KBr pellets for solids and thin films for liquids on a Shimadzo 8400S FTIR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Bruker AV III 500 MHz,Varian 400 MHz,or Varian 300 MHz spectrometer in CDCl3 or D2O. 1H NMR and 13C NMR chemical shifts (δ) in ppm are down field from tetramethylsilane. Elemental analyses were done in a Perkin Elmer CHNS/O Analyzer 2400.
General procedure for synthesis of catalyst: The catalyst was prepared with modification according to the procedures reported previously [13]. To a solution of 1-butyl-1,4-diazabicyclo[2.2.2]octan- 1-ium chloride (5.4 g,26.37 mmol) in dry acetonitrile,solid KOH (1.45 g,26.37 mmol) was added,and the mixture was stirred vigorously at room temperature for 15 h. The precipitate,KCl,was removed by filtration,and the resulting filtrate was evaporated at reduced pressure. The viscous liquid obtained was washed with diethyl ether (20 mL × 3) and dried to give pure ionic liquid, [C4dabco]OH (4.56 g,92.87%). 1HNMR(500 MHz,D2O): δ 3.3 (t,6H, J = 7.0 Hz),3.1 (t,2H,J = 9.0 Hz),3.0 (t,6H,J = 8.0 Hz),1.6 (quintet, 2H,J = 8.5 Hz),1.3-1.2 (m,2H),0.8 (t,3H,J = 7.5 Hz); 13C NMR (100 MHz,D2O): δ 64.4,52.0,44.2,23.1,19.1,12.8. Anal. Calcd. for: C10H22N2O: C,64.47; H,11.90; N,15.04. Found: C,64.37; H,11.78; N,14.91.
General procedure for Michael addition: To a well stirred mixture of active methylene compound 1 (1 mmol) and ionic liquid [C4dabco]OH (19.5 mg,5 mol% of the substrate),α,β-unsaturated carboxylic ester or nitrile 2 (2.1 mmol) was added and the reaction mixture was stirred at room temperature. The formation of the products was monitored by TLC. After completion of the reaction,water (2.0 mL) was poured to the reaction mixture, which was then filtered and dried to obtain the products. In general,no further purification was required to obtain the solid product. However,for the liquid mixture,ethyl acetate (2.0 mL) was added to the water extract of the reaction mixture. The organic phase was dried with anhydrous MgSO4 and evaporated. In some cases,the crude product was purified by column chromatography over silica gel to afford the pure product. All of the products were previously reported and were characterized by melting point determination,IR,and 1H NMR and 13C NMR spectroscopy. The ionic liquid catalyst was recovered from water and reused for the subsequent reactions. Selected data for typical compounds are given below.
Dimethyl-2,2-bis(2-cyanoethyl)malonate (3a): White solid,mp 141-142 ℃; IR (KBr,cm-1): v 2969,2253,1734,1466,1431,1205; 1H NMR (300 MHz,CDCl3): δ 3.8 (s,6H),2.4 (t,4H,J = 7.5 Hz),2.3 (t, 4H,J = 7.5 Hz); 13CNMR(75 MHz,CDCl3): δ 169.5,118.4,55.6,53.3, 29.8,13.1. Anal. Calcd. for C11H14N2O4: C,55.46; H,5.92; N,11.76. Found: C,55.42; H,5.98; N,11.61.
Diethyl bis(2-cyanoethyl)malonate (3d): White solid,mp 80- 81 ℃; IR (KBr,cm-1): v 2969,2253,1734,1468,1323,1209; 1H NMR (400 MHz,CDCl3): δ 4.2 (q,4H,J = 6.8 Hz),2.4 (t,4H, J = 7.6 Hz),2.2 (t,4H,J = 7.6 Hz),1.3 (t,6H,J = 7.2 Hz). 13C NMR (100 MHz,CDCl3): δ 170.0,119.0,58.6,54.6,28.3,22.8,13.7. Anal. Calcd. for C13H18N2O4: C,58.63; H,6.81; N,10.52. Found: C,58.47; H,6.68; N,10.31.
3,3-Diethyl-1,5-dimethylpentane-1,3,3,5-tetracarboxylate (3e): Colourless crystal,mp 40 ℃; IR (KBr,cm-1): v 2992,1730, 1443,1384,1304,1202,748; 1H NMR (400 MHz,CDCl3): δ 4.2 (q, 4H,J = 7.6 Hz),3.6 (s,6H),2.3 (t,4H,J = 8 Hz),2.2 (t,4H,J = 8 Hz), 1.2 (t,6H,J = 7.6 Hz). 13C NMR (100 MHz,CDCl3): δ 203.8,172.6, 171.1,61.5,61.4,51.5,28.5,26.4,26.2,13.5. Anal. Calcd. for C15H24O8: C,54.21; H,7.28. Found: C,54.10; H,7.12.
3-Ethyl-1,5-dimethyl-3-acetylpentane-1,3,5-tricarboxylate (3h): Colourless liquid; IR (KBr,cm-1): v 2995,1730,1712,1453, 1370; 1H NMR (300 MHz,CDCl3): δ 4.13 (q,2H),3.6 (s,6H),2.2-2.0 (m,11H),1.2 (t,3H,J = 7.2 Hz); 13C NMR (75 MHz,CDCl3): δ 204.0, 172.8,171.3,61.6,61.6,51.7,28.7,26.6,26.4,13.9. Anal. Calcd. for C14H22O7: C,55.62; H,7.33. Found: C,55.50; H,7.36.
Triethyl-3-acetylpentane-1,3,5-tricarboxylate (3i): Light yellow oil; IR (KBr,cm-1): v 2984,1741,1712,1550,1456,1371,1188, 1095,1024,860,783; 1H NMR (400 MHz,CDCl3): δ 4.2 (q,2H, J = 7.2 Hz),4.1 (q,4H,J = 7.2 Hz),2.3-2.1 (m,11H),1.3-1.2 (m,9H). 13C NMR (100 MHz,CDCl3): δ 172.6,171.1,61.5,51.6,28.6,26.5, 26.1,13.9. Anal. Calcd. for C16H2678: C,58.17; H,7.93. Found: C, 58.0; H,7.80.
Diethyl-4,4-diacetylheptanedioate (3l): White solid,mp 63- 64 ℃; IR (KBr,cm-1): v 2982,1732,1689,1367; 1HNMR(400 MHz, CDCl3): δ 4.1 (q,4H,J = 7.2 Hz),2.2 (t,4H,J = 7.2 Hz),2.14 (s,6H), 2.13 (t,4H,J = 7.2 Hz),1.3 (t,6H,J = 7.2 Hz). 13C NMR (100 MHz, CDCl3): δ 203.9,172.1,68.4,58.6,29.8,24.6,23.3,13.2. Anal. Calcd. for C15H24O6: C,59.98; H,8.05. Found: C,59.90; H,8.10. Dimethyl-4,4-dicyanoheptanedioate (3o): White solid,mp 64- 65 ℃; IR (KBr,cm-1): v 2963,2253,1732,1456,1375; 1H NMR (400 MHz,CDCl3): δ 3.7 (s,6H),2.7 (t,4H,J = 7.2 Hz),2.3 (t,4H, J = 8.4 Hz). 13C NMR (100 MHz,CDCl3): δ 171.2,115.1,62.9,27.8, 25.9,24.0,13.9. Anal. Calcd. for C311H12N2O4: C,58.63; H,6.81; N, 10.52. Found: C,58.53; H,6.85; N,10.39. Ethyl-2,4-dicyano-2-(2-cyanoethyl)butanoate (3q): Colourless liquid; 1H NMR (300 MHz,CDCl3): δ 4.3 (q,J = 7.2 Hz,2H),2.7-2.5 (m,4H),2.5-2.3 (m,2H),2.2-2.0 (m,2H),1.3 (t,J = 7.2 Hz,3H); 13C NMR (75 MHz,CDCl3): δ 166.2,117.5,116.4,64.4,47.8,32.2,14.2, 13.8. Anal. Calcd. for C13H19NO6: C,54.73; H,6.71; N,4.91. Found: C,54.58; H,6.80; N,4.79. 3-Ethyl-1,5-dimethyl-3-cyanopentane-1,3,5-tricarboxylate (3r): Colourless liquid; 1H NMR (300 MHz,CDCl3): δ 4.2 (q,2H, J = 7.2 Hz),3.6 (s,6H),2.6-2.0 (m,8H),1.3 (t,3H,J = 7.2 Hz); 13C NMR (75 MHz,CDCl3): δ 171.7,167.6,117.8,63.1,51.8,47.9,31.8, 29.8,13.9. Anal. Calcd. for C15H23NO6: C,57.50; H,7.40; N,4.47. Found: C,57.43; H,7.45; N,4.39. 3. Results and discussion
For optimization of the reaction conditions: first,the Michael addition of dimethylmalonate (1a) and acrylonitrile (2a) was carried out under solvent free conditions,in the presence of [C4dabco]OH as the catalyst. The reaction was very fast and the reaction mixture solidified as soon as the catalyst was added (entry 1,Table 1). The effect of the concentration of the catalyst was also studied (entries 1-3,Table 1). When the amount of the catalyst used was changed,we found that 5 mol% of the catalyst gave quantitative yield of the product (entry 3,Table 1). When the amount of the catalyst,[C4dabco]OH was further reduced to 1.0 and 0.5 mol%,the reaction required more time to complete; however,the yields were still very high. Next,the Michael addition reactions of 1a and 2a were studied by using various catalysts,such as,[C5dabco]OH,[C6dabco]OH,[C7dabco]OH,[C8dabco]OH, [C9dabco]OH and [C4dabco]Cl. It was found that the reaction proceeded efficiently,resulting in near a quantitative yield of the product and a slight change in reaction time (entries 4-9,Table 1). Thus,[C4dabco]OH (5 mol%) was taken as the catalyst of choice for the Michael addition reactions.
| Table 1 Michael addition of dimethylmalonate to acrylonitrile catalized by dabco-based ionic liquids.a |
The Michael addition reactions of various active methylene compounds to α,β-unsaturated carboxylic esters and nitriles were examined in the presence of [C4dabco]OH (5 mol%) under solvent free conditions,and the products were isolated with excellent yields (Table 2). All of the reactions given in Table 2 underwent bisaddition in one stroke within a very short duration with excellent yields. The formation of bis-adducts between different active methylene compounds and α,β-unsaturated compounds in the presence of several catalysts has been reported [9h, 10, 11]. However,there were some drawbacks of using these catalysts for the formation of bis-adducts in one step,such as,long reaction time [9h] low yields [9h] high reaction temperature [9h] large amount of catalyst [10],and use of volatile and toxic organic solvents [11]. Michael addition of acrylonitrile to different active methylene compounds in the presence of the catalyst [C4dabco]OH were faster than methylacrylate and ethylacrylate. This is because of the strong electron withdrawing nature of the cyano group compared to the carboxylic ester group. The product,which solidified from the reaction mixture in a few minutes,was filtered after the addition of water (2.0 mL),and no further purification was needed (entries 1,4,7,10,13,16,Table 2).
| Table 2 Michael addition catalyzed by [C4DABCO]OHa |
We also investigated the catalytic activities of [C4dabco]Cl alone and a combination of [C4dabco]Cl and [C4dabco]OH. However,the result obtained from combination of the catalysts was not better than the use of [C4dabco]OH alone.
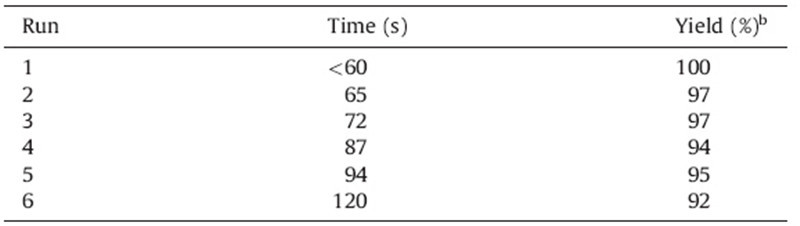
The recyclability of the catalyst was also examined (Table 3). Once product 3a had been filtered from the reaction mixture after addition of water (2.0 mL),excess water from the ionic liquid was evaporated under reduced pressure,and the catalyst was reused for the same reaction. The catalyst did not show much reduction of activity even after the sixth run. All reactions were completed in 1- 2 min affording 92%-100% yields.
| Table 3 Recycling of the catalyst [C4dabco]OH in the Michael addition of dimethylmalonate to acrylonitrile.a |
In conclusion,we have demonstrated that readily available DABCO-based ionic liquids behave as recyclable catalysts for the Michael addition of a broad range of activemethylene compounds with α,β-unsaturated carboxylic esters and nitriles,offering excellent yields in short durations. The reactions,without hazardous organic solvents,allowed Michael addition reactions to be performed in a simple,clean,and greenmanner.Most of the products required no further purification. In this process,after stirring for a few minutes,the product was isolated in pure form for both solid and liquid products. The catalyst was easily recovered and could be reusedmore than five timeswithout much loss of activity. What is more,only bis-addition products were detected. The procedure can be applied for large-scale syntheses also. Thus,we have developed an improved process which offers several advantages over other processes.Webelieve that this new synthetic method will greatly contribute to environmentally greener and safer processes.
AcknowledgmentsSanjoy Keithellakpam gratefully acknowledge the University Grants Commission,New Delhi for BSR-fellowship; and the authors acknowledge Guwahati University,the SAIF,IIT Madras and the CIF,IIT Guwahati,India for the spectral data.
| [1] | (a) T. Welton, Room-temperature ionic liquids. Solvents for synthesis and catalysis, Chem. Rev. 99 (1999) 2071-2084; (b) P. Wassercheid, W. Keim, Ionic liquids-new "solutions" for transition metal catalysis, Angew. Chem. Int. Ed. 39 (2000) 3772-3789; (c) R. Sheldon, Catalytic reactions in ionic liquids, Chem. Commun. (2001) 2399-2407; (d) J. Dupont, R.F. de Souza, P.A.Z. Suarez, Ionic liquid (molten salt) phase organometallic catalysis, Chem. Rev. 102 (2002) 3667-3692; (e) P. Wassercheid, T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim,2002. |
| [2] | J.A. Boon, J.A. Levisky, J.L. Pflug, J.S. Wilkes, Friedel-Crafts reactions in ambienttemperature molten salts, J. Org. Chem. 51 (1986) 480-483. |
| [3] | J.R. Harjani, S.J. Nara, M.M. Salunkhe, Lewis acidic ionic liquids for the synthesis of electrophilic alkenes via the Knoevenagel condensation, Tetrahedron Lett. 43 (2002) 1127-1130. |
| [4] | (a) J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J.P. Bazureau, J. Hamelin, Catalysed esterifications in room temperature ionic liquids with acidic counteranion as recyclable reaction media, Catal. Commun. 3 (2002) 185-190; (b) H. Xing, T. Wang, Z.H. Zhou, Y. Dai, Novel Brønsted-acidic ionic liquids for esterifications, Ind. Eng. Chem. Res. 44 (2005) 4147-4150; (c) J. Gui, X. Cong, D. Liu, et al., Novel brønsted acidic ionic liquid as efficient and reusable catalyst system for esterification, Catal. Commun. 5 (2004) 473-477; (d) H.P. Zhu, F. Yang, J. Tang, M.Y. He, Brønsted acidic ionic liquid 1-methylimidazolium tetrafluoroborate: a green catalyst and recyclable medium for esterification, Green Chem. 5 (2003) 38-39. |
| [5] | (a) G. Driver, K.E. Johnson, 3-Methylimidazolium bromohydrogenates(I): a roomtemperature ionic liquid for ether cleavage, Green Chem. 5 (2003) 163-169; (b) G.J. Kemperman, T.A. Roeters, P.W. Hilberink, Cleavage of aromatic methyl ethers by chloroaluminate ionic liquid reagents, Eur. J. Org. Chem. 9 (2003) 1681-1686. |
| [6] | (a) P. Formentin, H. Garcia, A. Leyva, Assessment of the suitability of imidazolium ionic liquids as reaction medium for base-catalysed reactions: case of Knoevenagel and Claisen-Schmidt reactions, J. Mol. Catal. A: Chem. 214 (2004) 137-142; (b) H. Chen, D.R. Justes, R.G. Cooks, Proton affinities of N-heterocyclic carbene super bases, Org. Lett. 7 (2005) 3949-3952. |
| [7] | (a) J.K. Myers, E.N. Jacobsen, Asymmetric synthesis of b-amino acid derivatives via catalytic conjugate addition of hydrazoic acid to unsaturated imides, J. Am. Chem. Soc. 121 (1999) 8959-8960; (b) J.S. Yadav, B.V.S. Reddy, G. Baishya, Green protocol for conjugate addition of thiols to α,β-unsaturated ketones using a [Bmim]PF6/H2O system, J. Org. Chem. 68 (2003) 7098-7100; (c) N. Azizi, M.R. Saidi, LiClO4 accelerated Michael addition of amines to a, b-unsaturated olefins under solvent-free conditions, Tetrahedron 60 (2004) 383-387. |
| [8] | (a) E.D. Bergmann, D. Ginsburg, R. Pappo, Organic Reactions, vol. 10, John Wiley & Sons, New York, 1959, p. 179; (b) S. Kobayashi, Rare earth metal trifluoromethanesulfonates as water-tolerant Lewis acid catalysts in organic synthesis, Synlett 9 (1994) 689-701; (c) N. Srivastava, B.K. Banik, Bismuth nitrate-catalyzed versatile Michael reactions, J. Org. Chem. 68 (2003) 2109-2114. |
| [9] | (a) L. Yang, L.W. Xu, C.G. Xia, Highly efficient KF/Al2O3-catalyzed versatile hetero- Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α,β-ethylenic compounds, Tetrahedron Lett. 46 (2005) 3279-3282; (b) D.Y. Kim, S.C. Huh, S.M. Kim, Enantioselective Michael reaction of malonates and chalcones by phase-transfer catalysis using chiral quaternary ammonium salt, Tetrahedron Lett. 42 (2001) 6299-6301; (c) A. Horvath, Catalysis and regioselectivity in the Michael addition of azoles. Kinetics vs. thermodynamic control, Tetrahedron Lett. 37 (1996) 4423-4426; (d) K. Shimizu, M. Miyagi, T. Kan-no, T. Kodama, Y. Kitayama, Fe3+-exchanged fluorotetrasilicic mica as an active and reusable catalyst for Michael reaction, Tetrahedron Lett. 44 (2003) 7421-7424; (e) B. Ni, Q. Zhang, A.D. Headley, Functionalized chiral ionic liquid as recyclable organocatalyst for asymmetric Michael addition to nitrostyrenes, Green Chem. 9 (2007) 737-739; (f) M.M. Dell'Anna, V. Gallo, P. Mastrorilli, et al., Metal catalysed Michael additions in ionic liquids, Chem. Commun. (2002) 434-435; (g) X. Xin, X. Guo, H.F. Duan, Y. Lin, H. Sun, Efficient Knoevenagel condensation catalyzed by cyclic guanidiniumlactate ionic liquid as medium, Catal. Commun. 8 (2007) 115-117; (h) G.A. Salvador, S. Hasegawa, M. Hirano, S. Komiya, Michael reaction promoted byη1-o-enolatoruthenium(Ⅱ) complexes derived from Ru(cod)(cot), diphosphine, and dimethyl malonate, Tetrahedron Lett. 39 (1998) 5209-5212. |
| [10] | B.C. Ranu, B. Subhash, Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in Michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles, Org. Lett. 7 (2005) 3049-3052. |
| [11] | Y. Yu, L.B. Wang, Z. Zhang, et al., [Bmim]OAc catalyzed Michael addition of active methylene to α,β-unsaturated carboxylic esters and nitriles, Chem. Res. Chin. Univ. 26 (2010) 554-557. |
| [12] | Y. Yang, S.X. Dong, X.H. Liu, L.L. Lin, X.M. Feng, Chiral guanidine-catalyzed asymmetric direct vinylogous Michael reaction of a, b-unsaturated γ-butyrolactams with alkylidene malonates, Chem. Commun. 48 (2012) 5040-5042. |
| [13] | Z.Z. Yang, L.N. He, X.Y. Dou, S. Chanfreau, Dimethyl carbonate synthesis catalyzed by DABCO-derived basic ionic liquids via transesterification of ethylene carbonate with methanol, Tetrahedron Lett. 51 (2010) 2931-2934. |