b State Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, Chengdu 610054, China
1. Introduction
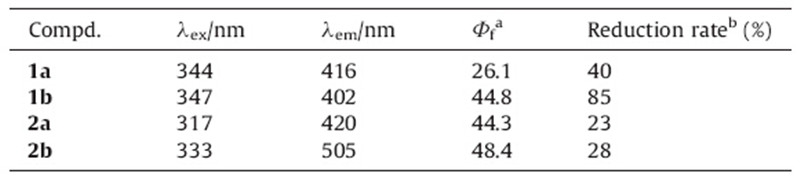
Photochromic compounds have received increasing attention in recent years due to their potential application in optical data storage and photo-controllable materials [1, 2, 3, 4, 5, 6, 7, 8, 9]. In particular, fluorescent photochromic bisthienylethenes currently have been extensively explored for use as chemosensors and in biological imaging [10, 11, 12]. There are many ways to design fluorescent photochromic bisthienylethenes,including linking fluorophores on the branched chains of the aryl [13, 14, 15],and using the fluorophores as the center ethene bridge [16, 17]. Triaryl(heteroaryl)- imidazoles are one kind of classic chromophores,which are usually used in the field of photochromic compounds because of their fine optoelectronic properties [18, 19, 20, 21]. Herein,we synthesized a series of bisthienylethenes containing imidazole and imidazolium derivatives,and their photochromic and fluorescent switch properties were investigated (Scheme 1).
|
Download:
|
| Scheme 1.Photochromism of diarylethenes 1a,1b and 2a,2b. | |
N-(4-Formylphenyl)-aza-15-crown-5-ether 3 and diketone 5 were synthesized according to the literature [22, 23]. Other reagents were commercially available and used without further purification. 1H NMR and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer in DMSO-d6 solutions. High resolution mass spectra (HRMS) were recorded on a Waters LCT Premier XE spectrometer using standard conditions (ESI,70 eV). UV-vis absorption spectra were measured on a Varian Cary 500 UV-vis spectrophotometer. Fluorescence emission spectra were measured on a Varian Cary Eclipse Fluorescence spectrophotometer. Fluorescence quantum yields were recorded on a Fluoro Max-4 spectrofluorometer with a quanta-Φ integrating sphere (Horiba Jobin-Yvon). 2.2. Synthesis of diarylethene 1a
A mixture of formyl azacrown ether 3 (0.96 mmol),ammonium acetate (10 equiv.) and diketone 5 (0.96 mmol) in glacial acetic acid (50 mL) was stirred and heated at reflux for 12 h. Then the mixture was cooled to room temperature and the product precipitated during neutralization with 5 mol L-1 NH4OH. The crude product was purified through column chromatography on silica using ethyl acetate/petroleum ether (3:1) as the eluent. Compound 1a was separated in 38% yield as a yellow solid. Mp 122.1-124.0 ℃. 1H NMR (400 MHz,DMSO-d6): δ 12.09 (s,1H),7.80 (d,2H,J = 8.90 Hz),6.71 (d,2H,J = 7.60 Hz),6.68 (s,1H),6.57 (s, 1H),3.66 (t,4H,J = 6.00 Hz),3.60-3.46 (m,16H),2.41 (s,3H),2.33 (s,3H),2.11 (s,3H),1.96 (s,3H). 13C NMR (101 MHz,DMSO-d6): δ 144.71,135.95,127.76,126.75,111.35,70.29,69.53,69.01,67.74, 51.95,14.79,13.40. ESI-HRMS (m/z): Calcd. for C31H40N3O4S2 ([M+H]+): 582.2460; Found: 582.2465. 2.3. Synthesis of diarylethene 1b
Compound 1b was prepared by an analogous method to that used for 1a and obtained as a white solid in a yield of 25%. Mp 246.7-247.4 ℃. 1H NMR (400 MHz,DMSO-d6): δ 12.36 (s,1H),7.91 (d,2H,J = 8.70 Hz),7.36-7.30 (m,4H),7.10-6.99 (m,8H),6.72 (s, 1H),6.56 (s,1H),2.41 (s,3H),2.32 (s,3H),2.13 (s,3H),1.97 (s,3H). 13C NMR (101 MHz,DMSO-d6): δ 147.00,146.92,144.76,135.16, 134.66,134.06,133.50,132.13,131.25,129.60,129.62,128.06, 127.22,126.82,126.11,124.77,124.14,123.29,122.88,14.83, 13.86,13.50. ESI-HRMS (m/z): Calcd. for C33H30N3S2 ([M+H]+): 532.1881; Found: 532.1887. 2.4. Synthesis of compound 2a
Iodomethane (0.54 mmol) was added to a mixture of 1a (210 mg,0.36 mmol) and K2CO3 (100 mg,0.72 mmol) in N,Ndimethylformamide (15 mL),then the mixture was heated under nitrogen atmosphere and stirred at 50 ℃ for 4 h. The solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate and washed with water and brine. Removal of the solvent led to the isolation of an off-white solid. The solid was dissolved in acetonitrile (7 mL),and iodomethane (0.34 mL) was added to this solution. The mixture was heated to reflux for 24 h. The solvent was removed under reduced pressure,and the residue was washed with diethyl ether. The resulting solid was dissolved in acetonitrile (5 mL),and then saturated NH4PF6 solution was added to the mixture until the precipitate appeared. The solid was collected by filtration and washed with deionized water. The final product was obtained as a white solid in a yield of 51%. Mp 249.2- 250.2 ℃. 1H NMR (400 MHz,CDCl3): δ 7.53 (d,1H,J = 8.10 Hz),6.86 (d,1H,J = 8.60 Hz),6.80 (s,0.64H),6.57 (s,0.34H),3.80 (t,2H, J = 6.00 Hz),3.73-3.63 (m,8H),3.54 (s,3H),2.43 (s,3H),2.22 (s, 1H),1.94 (s,2H). 13C NMR (101 MHz,DMSO-d6): δ 149.99,137.18, 131.65,126.67,122.12,111.32,70.25,69.57,68.86,67.59,52.08, 34.23,14.87,13.33. ESI-HRMS (m/z): Calcd. for C33H44N3O4S2 ([M+H]+): 610.2773; Found: 610.2773. 2.5. Synthesis of compound 2b
Compound 2b was prepared by a method similar to that used for 2a and obtained as a white powder in a yield of 52%. Mp 275.1- 276.0 ℃. 1H NMR (400 MHz,DMSO-d6): δ 7.65 (d,1H,J = 8.10 Hz), 7.44 (t,2H,J = 7.80 Hz),7.20-7.31 (m,3H),7.07 (d,1H,J = 8.80 Hz), 6.90 (s,0.61H),6.64 (s,0.36H),3.49 (s,3H),2.42 (s,3H),2.18 (s,1H), 1.93 (s,2H). 13C NMR (101 MHz,DMSO-d6): δ 150.65,145.74, 137.32,131.72,130.06,126.95,126.18,125.36,121.98,118.90, 34.14,14.87,13.35. ESI-HRMS (m/z): Calcd. for C35H34N3S2 ([M+H]+): 560.2194; Found: 560.2191. 3. Results and discussion 3.1. Synthetic strategy and the X-ray structures of 1a
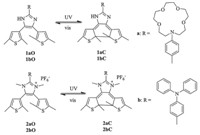
In this work,a series of bisthienylethenes containing imidazole and imidazolium derivatives were prepared by a simple method, and their photochromic behaviors were investigated in detail. The synthetic route used to obtain diarylethene 1 and 2 is shown in Scheme 2. N-(4-Formylphenyl)-aza-15-crown-5-ether 3 and diketone 5 were synthesized according to the literatures. Treatment of diketone in acetic acid with corresponding aldehyde in the presence of NH4Ac afforded target compounds 1a and 1b in yields of 20%-40%. Compound 2 was prepared by compound 1 methylating with CH3I,and then ion exchanging with NH4PF6. All of the target compounds were characterized by 1H NMR,13C NMR and HRMS,and the corresponding spectra were shown in Figs. S1-S12 in Supporting information. It is worth mentioning that the single crystal of 1a was obtained by slow evaporation in THF. The structure of compound 1a was further determined by X-ray diffraction (Fig. 1),the distance between the reactive carbons (C5- C10) is 3.71Å for 1a,which is short enough to undergo photochromic reactions in the single-crystalline phase [24]. Crystal determination data of 1a was shown in Table S1 in Supporting information with CCDC number 969060.

|
Download:
|
| Scheme 2.Synthetic route for the target compounds. | |
|
Download:
|
| Fig. 1.Molecular structure of 1a (H atoms have been omitted for clarity). | |
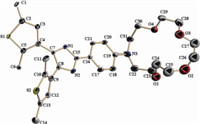
Bisthienylethenes 1 and 2 can undergo a reversible photochromic reaction in acetonitrile upon alternating irradiation with UV and visible light. Changes in the absorption spectra of compound 1 and 2 induced by photo irradiation at room temperature in acetonitrile (1 × 10-5 mol L-1) are shown in Fig. 2. The maximum absorption of compound 1a is observed at 344 nm. Upon irradiation with 365 nm light,the colorless solution of 1aO gradually turns purple due to the appearance of a new visible absorption band centered at 550 nm,which is attributed to the formation of the closed-ring form 1aC. The photo-stationary state is attained after about 20 s. Alternatively,the purple solution could be bleached to colorlessness upon irradiation with visible light (λ > 550 nm),indicating that 1aC returned to the initial state 1aO. Compared to 1aO,the maximum absorption of compound 2aO appeared at 318 nm. And the maximum absorption of ring-closed isomer 2aC appeared at 576 nm,which is a 26 nm bathochromic shift from 1aC. The possible reason is that the methylation of the imidazole increased the π-conjugation of the ring-closed isomer 1aC. Similarly,the colorless solutions of 1b and 1b can turn purple on irradiation with 365 nm UV light,and the color can also be disappeared upon irradiation with the same visible light (λ > 550 nm). The corresponding spectra changes are shown in Fig. 2b and 2d. The photochromic properties of diarylethenes 1a, 1b and 2a,2b are summarized in Table 1. The thermal stabilities of these diarylethenes were also investigated. After putting the solutions of open-ring isomers of diarylethenes 1a,1b and 2a,2b in acetonitrile at room temperature in the dark for more than one week,no changes in their colors and spectra were observed. The color of the closed-ring isomers of 1a and 1b were bleached after staying in the dark for several minutes. However,the color of the closed-ring isomers of 2a and 2b would not change even after several hours,which means that the N-methylation of the imidazole ring can improve the thermal stability of these diarylethenes with the imidazole ring.
| Table 1 Absorption parameters and photochromic reactivities of diarylethenes 1a,1b,2a,and 2b in acetonitrile. |
|
Download:
|
| Fig. 2.Changes in the absorption spectra of compounds 1a (a),1b (b),2a (c),and 2b (d) in acetonitrile at room temperature (1 × 10-5 mol L-1). | |
The fluorescence spectra changes of the diarylethene 1 and 2 in acetonitrile at room temperature are shown in Fig. 3. In acetonitrile,diarylethene 1aO exhibited strong blue emitting fluorescence at 416 nm when excited at 344 nm. After irradiation with 365 nm UV light,the fluorescence intensity of 1a decreased to 40% due to the formation of ring-closed isomer. Similar results were obtained when solutions of the other bisthienylethenes 1b, 2a,and 2b in acetonitrile were irradiated with UV light. The corresponding fluorescence data of these compounds is summarized in Table 2.
| Table 2 Fluorescence data of diarylethenes 1a,1b,2a,and 2b in acetonitrile (1×10-5 mol L-1). |
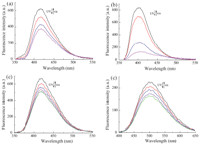
|
Download:
|
| Fig. 3.Fluorescent changes of compounds 1a (a),1b (b),2a (c),and 2b (d) upon alternate irradiation with UV/vis light in acetonitrile (1 × 10-5 mol L-1) at room temperature. | |
As shown in Table 2,the different substituents and the methylation of the imidazole ring have a great influence on the fluorescent performances of the bisthienylethenes. Compared to 1b,the fluorescence emission wavelength of 1a was 14 nm redshifted due to the difference between the N-(4-phenyl)-aza-15- crown-5-ether and the triphenylamine groups. After the methylation of the imidazole,the fluorescence emission wavelength of 2a was 4 nm bathochromic shifted from 1a because the methylation extended the π-conjugation. A much longer bathochromic shift was observed from 2a to 2b,which can reach about 103 nm. In this way,the fluorescence emission colors could be easily tuned from blue to yellow-green,which were shown in Fig. 4. Moreover,the fluorescence quantum yields of these bisthienylethenes could be regulated from 26.1% to 48.4%,which provided a possible application for biological imaging and cell staining. According to the above analysis,the light-emitting properties of this kind of diarylethenes could be manipulated by easy modification of the imidazole group.

|
Download:
|
| Fig. 4.Fluorescence emission of compounds 1aO,1bO,2aO,and 2bO (from left to right) by photo irradiation in acetonitrile (2 × 10-5 mol L-1) at room temperature. | |
A series of novel bisthienylethenes containing imidazole and imidazolium derivatives have been prepared,and the crystal structure of compound 1a was obtained. Their photochromism and fluorescent properties in solution were investigated. It has been demonstrated that the fluorescent emission wavelength of the photochromic bisthienylethenes could be simply tuned by varying the imidazole groups,which provides a simple solution for designing the fluorescent photochromic diarylethenes.
AcknowledgmentsThis work was financially supported by National Basic Research 973 Program (No. 2013CB733700),National Natural Science Foundation of China (Nos. 21190033,21302056 and 21072058) and the Open Foundation of State Key Laboratory of Electronic Thin Films and Integrated Devices (No. KFJJ201311). Professor He Tian is also acknowledged for helpful discussion.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.033.
| [1] | M. Irie, Diarylethenes for memories and switches, Chem. Rev. 100 (2000) 1685-1716. |
| [2] | H. Tian, S.J. Yang, Recent progresses on diarylethene based photochromic switches, Chem. Soc. Rev. 33 (2004) 85-97. |
| [3] | J.J. Zhang, Q. Zou, H. Tian, Photochromic materials: more than meets the eye, Adv. Mater. 25 (2013) 378-399. |
| [4] | J.Q. Zhang, J.Y. Jin, L. Zou, H. Tian, Reversible photo-controllable gels based on bisthienylethene-doped lecithin micelles, Chem. Commun. 49 (2013) 9926-9928. |
| [5] | C.R. Zhang, W.P. Yan, M.G. Fan, Synthesis and photoreaction mechanism of a novel bifunctional photochromic compound, Chin. Chem. Lett. 18 (2007) 519-522. |
| [6] | Z.Y. Li, W. Xue, G.X. Liu, et al., Synthesis and properties of template-promoted switchabledithienylethene-basedmacrocycles, Chin.Chem. Lett. 24 (2013)189-191. |
| [7] | G.Y. Jiang, S. Wang, W.F. Yuan, et al., Photo- and proton-dual-responsive fluorescence switch based on a bisthienylethene-bridged naphthalimide dimer and its application in security data storage, Eur. J. Org. Chem. 13 (2007) 2064-2067. |
| [8] | S. Pu, C. Zheng, Q. Sun, G. Liu, C. Fan, Enhancement of cyclization quantum yields of perfluorodiarylethenes via weak intramolecular interactions, Chem. Commun. 49 (2013) 8036-8038. |
| [9] | G. Liu, S.Z. Pu, R.J. Wang, Photochromism of asymmetrical diarylethenes with a pyrrole unit: effects of aromatic stabilization energies of aryl rings, Org. Lett. 15 (2013) 980-983. |
| [10] | Q. Zou, X. Li, J. Zhang, et al., Unsymmetrical diarylethenes as molecular keypad locks with tunable photochromism and fluorescence via Cu2+ and CN- coordinations, Chem. Commun. 48 (2012) 2095-2097. |
| [11] | J.Q. Zhang, J.Y. Jin, J.J. Zhang, L. Zou, An optic/proton dual-controlled fluorescence switch based on novel photochromic bithienylethene derivatives, Chin. J. Chem. 30 (2012) 1741-1747. |
| [12] | U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie, N.R. Branda, A photocontrolled molecular switch regulates paralysis in a living organism, J. Am. Chem. Soc. 131 (2009) 15966-15967. |
| [13] | S.Z. Pu, D.H. Jiang, W.J. Liu, G. Liu, S.Q. Cui, Multi-addressable molecular switches based on photochromic diarylethenes bearing a rhodamine unit, J. Mater. Chem. 22 (2012) 3517-3526. |
| [14] | J.J. Zhang, W.J. Tan, X.L. Meng, H. Tian, Soft mimic gear-shift with a multi-stimulus modified diarylethene, J. Mater. Chem. 19 (2009) 5726-5729. |
| [15] | S.Z. Pu, Z.P. Tong, G. Liu, R.J. Wang, Multi-addressable molecular switches based on a new diarylethene salicylal Schiff base derivative, J. Mater. Chem. C 1 (2013) 4726-4739. |
| [16] | W.H. Zhu, Y.H. Yang, R. Métivier, et al., Unprecedented stability of a photochromic bisthienylethene based on benzobisthiadiazole as an ethene bridge, Angew. Chem. Int. Ed. 50 (2011) 10986-10990. |
| [17] | X.L. Meng, W.H. Zhu, Q. Zhang, et al., Novel bisthienylethenes containing naphthalimide as the center ethene bridge: photochromism and solvatochromism for combined NOR and INHIBIT logic gates, J. Phys. Chem. B 112 (2008) 15636-15645. |
| [18] | E. Oliveira, R.M. Baptista, S.P. Costa, M.M. Raposo, C. Lodeiro, Exploring the emissive properties of new azacrown compounds bearing aryl, furyl, or thienyl moieties: a special case of chelation enhancement of fluorescence upon interaction with Ca2+, Cu2+, or Ni2+, Inorg. Chem. 49 (2010) 10847- 10857. |
| [19] | G.P. Duan, N.Y. Zhu, V.W. Yam, Syntheses and photochromic studies of dithienylethene- containing imidazolium derivatives and their reactivity towards nucleophiles, Chem. Eur. J. 16 (2010) 13199-13209. |
| [20] | Z. Li, J. Xia, J. Liang, et al., Synthesis of diarylethene derivatives containing various heterocycles and tuning of light-emitting properties in a turn-on fluorescent diarylethene system, Dyes Pigments 90 (2011) 290-296. |
| [21] | H.H. Liu, Y. Chen, Selective photoconversion of photochromic diarylethenes and their properties, New J. Chem. 36 (2012) 2223-2227. |
| [22] | M.P. Vladimirova, S.D. Simova, E.R. Stanoeva, M.I. Mitewa, Synthesis and spectroscopic properties of new Schiff bases containing the N-phenylaza-15-crown-5 moiety, Dyes Pigments 50 (2001) 157-162. |
| [23] | L. Belen'kii, V. Shirinyan, G. Gromova, et al., A new approach to the synthesis of dithienylethanediones and dithienylacetylenes, Chem. Heterocycl. Compd. 39 (2003) 1570-1579. |
| [24] | S. Nakamura, M. Irie, Thermally irreversible photochromic systems. A theoretical study, J. Org. Chem. 53 (1988) 6136-6138. |