1. Introduction
With the diminishing of fossil fuels,the search for sustainable, alternative energy has become critically important in recent decades. Increasing interest has been devoted to the selective dehydration of hexose to 5-hydroxymethylfurfural (HMF) [1, 2],as HMF and its derivatives have potential applications in the production of fine chemicals,pharmaceuticals,plastics [3],and liquid alkanes [4]. Presently,HMF is formed with high yield by the acid-catalyzed dehydration of fructose [5, 6]. However,the key problem with the use of fructose is its high cost. One of the most important starting chemicals from biomass is glucose. With its low cost and wide supply,the conversion of glucose to HMF has attracted much attention [7, 8, 9].
For the dehydration of glucose,the selection of catalysts is very important. Several kinds of catalysts,such as HCl,H2SO4 [10, 11], CrCl3-hydroxyapatite [9],and TiO2 [12] have been investigated. However,the catalysts mentioned above have some disadvantages, such as pollution,separation,and recycling problems. The development of environmental friendly catalysts with high activity is expected. In addition,the conversion of monosaccharides has been carried out in some polar organic solvents,dimethylsulfoxide [13],dimethylformamide [14],or acetone [11],and sub-critical or high temperature water [15, 16]. However,this approach necessitates difficult and energy-intensive isolation procedures. Therefore, an efficient dehydration system is greatly needed.
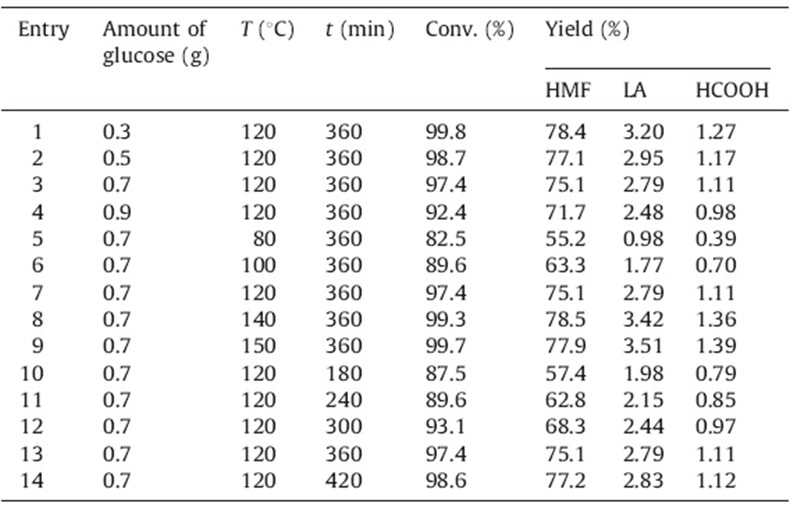
Room temperature ionic liquids (ILs),which could dissolve carbohydrates effectively [17, 18, 19],have attracted much attention in recent years. In pure water,the dehydration of monosaccharide is generally nonselective,leading to many by-products besides HMF [20]. Biphasic systems,in which a water-immiscible organic solvent is added to extract continuously the HMF from the aqueous phase,have also been investigated [21]. In our previous work [22], we studied the conversion of xylose to furfural with an acidic ionic liquid. Here,we describe an efficient method for the dehydration of glucose into HMF with a biphasic system and SO3H-functionalized ionic liquids as catalysts. The HMF production from glucose is shown in Scheme 1.
|
Download:
|
| Scheme 1.A typical reaction scheme for HMF production from glucose. | |
D-Glucose (98%) was a commercial product from Sinopharm Chemical Reagent Co.,Ltd.; 5-HMF (>99%) was from Aldrich; 4- methyl-2-pentanone (AR,>90%),formic acid (AR,>98%),and acetonitrile (HPLC) were purchased from Tianjin chemical reagent company (Tianjin,China); 1-methyl imidazole,1-butyl imidazole, 1-vinyl imidazole,and 1,4-butanesultone were from Alfa Aesar and all of them were CP grade (>99%); levulinic acid was purchased from DaoCheng chemical company (Hong Kong,China); all other reagents and solvents were commercially available and used without further purification. 2.2. Typical procedure for synthesis of SO3H-functionalized ionic liquids
The ionic liquids used in this study (Fig. 1) were synthesized as described in the literature [23, 24]. IL-1,for example,was prepared as follows: 1-methylimidazole (16.4 g,0.2 mol) and 1,4-butanesultone (27.28 g,0.2 mol) were mixed in a 100 mL round bottom flask. The mixture was stirred at 42-45 ℃ for 17 h. The white solid zwitterion was washed repeatedly with ether to remove non-ionic residues,filtrated through a Buchner funnel,and dried in vacuum for 4 h. A stoichiometric amount of trifluoroacetic acid (22.8 g, 0.2 mol) was added dropwise,and the mixture was stirred for 6 h at 80 ℃. The viscous liquid was washed three times with ether and dried in vacuum to form IL-1. 1H NMR (400 MHz,CDCl3): δ 1.197- 1.215 (m,2H),1.232-1.245 (m,2H),1.739 (t,2H),3.490 (s,3H), 3.880 (t,2H),7.436 (s,1H),7.541 (s,1H),9.026 (s,1H); 13C NMR (100 MHz,CDCl3): δ 21.544,27.932,36.121,48.857,50.074, 116.042 (1JCF = 289.3 Hz),120.274,122.093,123.902,135.579, 162.157 (2JCF = 36.6 Hz). ESI-MS: m/z (+) 218.8,m/z (-) 112.7.

|
Download:
|
| Fig. 1.Ionic liquids used in this study. | |
The synthesis of ionic liquids IL-2 to IL-8 followed the same protocol as was used for the preparation of IL-1. Their NMR and ESI-MS spectral characteristics are shown as follows:
IL-2: 1H NMR (400 MHz,D2O): δ 1.664-1.741 (m,2H),1.947- 2.046 (m,2H),2.903 (t,2H),3.852 (s,3H),4.210 (t,2H),7.400 (s,1H),7.462 (s,1H),8.707 (s,1H); 13C NMR (100 MHz,D2O): δ 20.877,28.055,35.607,48.865,50.009,122.120,123.606,135.934, 176.615. ESI-MS: m/z (+) 218.8,m/z (-) 58.9.
IL-3: 1H NMR (400 MHz,D2O): δ 1.615-1.668 (m,2H),1.875- 1.948 (m,2H),2.832 (t,2H),3.783 (s,3H),4.135 (t,2H),7.329 (s,1H),7.389 (s,1H),8.627 (s,1H); 13C NMR (100 MHz,D2O): δ 20.837,28.010,35.580,48.823,49.978,122.076,123.569,135.871. ESI-MS: m/z (+) 218.8,m/z (-) 96.5
IL-4: 1H NMR (400 MHz,D2O): δ 1.568-1.645 (m,2H),1.826- 1.901 (m,2H),2.260 (s,3H),2.813 (t,2H),3.735 (s,3H),4.074 (t,2H),7.227 (d,2H),7.273 (s,1H),7.331 (s,1H),7.555 (d,2H), 8.563 (s,1H); 13C NMR (100 MHz,D2O): δ 20.406,20.852,28.010, 35.560,48.810,49.985,122.049,123.551,125.252,129.353, 135.775,139.435,142.334. ESI-MS: m/z (+) 218.8,m/z (-) 170.7.
IL-5: 1H NMR (400MHz,CDCl3 ): δ 0.731-0.856 (m,2H), 0.942-0.997 (m,2H),1.892 (t,2H),2.809 (s,3H),3.146 (t,2H), 7.361 (s,1H),7.384 (s,1H),8.517 (s,1H); 13C NMR (100 MHz, CDCl3): δ 20.612,28.207,36.114,49.031,50.698,118.444 (JCF = 224.3 Hz),122.477,123.948,136.025. ESI-MS: m/z (+) 218.9,m/z (-) 148.6.
IL-6: 1H NMR (400 MHz,D2O): δ 1.531-1.607 (m,2H),1.802- 1.915 (m,2H),2.724 (t,2H),3.675 (s,3H),4.028 (t,2H),7.179 (s, 1H),7.242 (s,1H),8.471 (s,1H); 13C NMR (100 MHz,D2O): δ 20.907,28.204,35.851,49.063,50.258,122.336,123.719,136.018. ESI-MS: m/z (+) 218.6,m/z (-) 96.3.
IL-7: 1H NMR (400 MHz,CDCl3): δ 0.643-0.697 (m,2H),0.842- 0.913 (m,2H),1.255 (t,1H),1.884 (t,2H),2.252 (d,2H),3.166 (t, 2H),7.342 (s,1H),7.742 (s,1H),8.724 (s,1H); 13C NMR (100 MHz, CDCl3): δ 23.016,23.769,29.134,35.735,48.414,74.063,122.198, 122.374,135.107. ESI-MS: m/z (+) 230.9,m/z (-) 96.6.
IL-8: 1H NMR (400 MHz,D2O): δ 0.766 (t,3H),1.114-1.207 (m, 2H),1.554-1.632 (m,2H),1.669-1.865 (m,2H),1.884-1.921 (m, 2H),2.794 (t,2H),4.056 (t,2H),4.106 (t,2H),7.364 (s,1H),7.379 (s, 1H),8.670 (s,1H); 13C NMR (100 MHz,D2O): δ 12.536,18.664, 20.862,28.009,31.094,48.859,49.266,49.980,122.198,122.417, 135.115. ESI-MS: m/z (+) 260.9,m/z (-) 96.6. 2.3. Typical procedure for glucose dehydration
The as-received glucose was dried for 24 h at 90 ℃ prior to dehydration reaction. Experiments were carried out in a Teflonlined stainless steel autoclave equipped with a heating jacket. After the catalysts (typically 0.3 g) and glucose (0.7 g) were added into the autoclave pre-charged with water and 4-methyl-2-pentanone (MIBK) (typically 8 mL),the reaction was started under spontaneous pressure by heating the mixture to the reaction temperature. After the reaction,the reactor was removed and quickly quenched in a cool water bath. Subsequently,filtration,extraction,and separation were conducted; and then the organic and aqueous phases were collected to characterize the products. For the recycling of IL-5,HMF was extracted out from the water phase 5 times with 8 mL of ethyl acetate,after extraction,the aqueous phase was heated at 60 ℃ for 24 h in a vacuum oven to remove water and residual ethyl acetate. The IL-5 was then used directly for the next run by adding glucose and MIBK. All results were replicated at least three times. 2.4. Analyses
After each dehydration run,the consumption of glucose was confirmed by the phenol-sulfuric acid method [25]. A mixture containing 0.1 mL reaction samples (water-soluble portion), 0.9 mL deionized water,1 mL 5% phenol (freshly distilled),and 5 mL 98% concentrated sulfuric acid was prepared. The analysis was performed on an HP 8453 UV-vis spectrophotometer at about 490 nm with a slit width of 0.06 mm. The concentration of glucose in the reacted solution was calculated based on the standard curve obtained with glucose.
Analysis of HMF was performed by HPLC on an HP 1090 series equipped with a photodiode array UV detector and a zorbax eclipse plus C18 reversed-phase column (150 mm × 4.6 nm,0.5 μm). During this process,the column temperature remained constant at 30 ℃,while the mobile phase applied was water-acetonitrile (15:85,v/v) at the flow rate of 0.5 mL/min with UV detection at 280 nm.
In addition,for the characterization of ionic liquids,NMR spectra were recorded on an INOVA-400 spectrometer; ESI-MS analyses were performed by using a Waters micromass ZQ alliance spectrometer. The Hammett acidity function of ionic liquids was recorded on an HP 8453 UV-vis spectrophotometer. 3. Results and discussion 3.1. Glucose dehydration in SO3H-functionalized ionic liquids
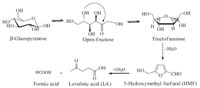
With increasing concerns on environmental protection and sustainable development,ionic liquids as catalysts were studied in our work. Fig. 2 shows the dehydration of glucose in eight SO3H-functionalized ionic liquids. The catalysts were efficient for glucose dehydration,and the acidity of the ionic liquid had large effects on the reaction. The nature of the anion influence on the catalytic activity of such SO3H-functionalized ionic liquids is well known. Ionic liquids with anions p-CH3C6H4SO3-,CF3SO3-,and HSO4- (IL- 4 to IL-8) resulted in high yields of HMF. When IL-5 was used as catalyst,glucose conversion was 97.4% and HMF yield reached 75.1%. However,SO3H-functionalized ionic liquids with anions CF3COO- and CH3COO- (IL-1 and IL-2) showed remarkably poor activities,perhaps because of the weak acidity of trifluoroacetic acid and acetic acid. Phosphoric acid as anion (IL-3) showed slightly lower activity than IL-6,most probably because of its weaker acidity,too. The Hammett acidity functions (H0) of the eight ionic liquids were calculated (not shown),which suggested that the order of acidity reflected the catalytic activities of ionic liquids.
|
Download:
|
| Fig. 2.Glucose dehydration in various SO3H-functionalized ionic liquids (0.7 g glucose,0.2 g ionic liquids,1 mL H2O,8 mL MIBK,T = 120 ℃,t = 360 min, P = 2.5 atm.). | |
Collectively,the results above indicated that strong acidity of the ionic liquid was an important aspect in glucose dehydration. Meanwhile,the structure of ionic liquids was also investigated. Fig. 2 shows that while the anion was HSO4- and position 1 in the imidazole ring was occupied by methyl,ethylene,n-C4H9 (IL-6 to IL-8,respectively),the results of glucose dehydration were almost equivalent,which means that the influence of an ionic liquid’s structure on dehydration reaction was not as significant as that of its acidity. Therefore,we could confirm that the acidity of the solution had a large effect on glucose dehydration into HMF. 3.2. Influence of IL-5 dosage on glucose dehydration
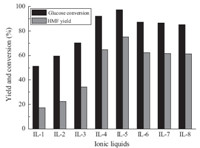
As IL-5 was the most effective catalyst in our study,we chose IL- 5 as the probe to investigate the influence of reaction conditions. Fig. 3 shows the effects of IL-5 dosage on glucose conversion and HMF yield. The amounts of IL-5 used were 0.1 g,0.2 g,0.3 g,0.4 g, 0.5 g. In the absence of IL-5,at a reaction temperature of 120 ℃,no HMF yield was observed for 120 min reaction time,and the conversion of glucose was only 7.8% (not shown). As shown in Fig. 3,when the dosage of IL-5 was 0.1 g,the glucose conversion reached 93.9% and HMF yield was 68.1%; while the IL-5 amount increased from 0.1 g to 0.3 g,the yield of HMF increased from 68.1% to 76.9%,and glucose conversion was up to 99.8%; however,when the dosage of IL-5 increased from 0.3 g to 0.5 g,both glucose conversion and HMF yield decreased. We considered that when IL- 1 dosage was above 0.3 g,side effects occurred,such as aldol condensation and rehydration of HMF,which led to the decrease of HMF selectivity and glucose conversion. The HMF yield did not increase with IL-5 amounts over 0.3 g,which implied that there were sufficient catalytic sites available for the substrate glucose (0.7 g) in our system at the experimental conditions.
|
Download:
|
| Fig. 3.Influence of IL-5 dosage on glucose dehydration (0.7 g glucose,1 mL H2O, 8 mL MIBK,T = 120 ℃,t = 360 min,P = 2.5 atm.). | |
Generally,in the dehydration of glucose,in the first step,the reaction of glucose-fructose isomerization occurred,and then fructose dehydrated into HMF,as indicated in Scheme 1. Kuster et al. [26] demonstrated that in the dehydration of fructose,the product HMF can combine with fructose and cross-polymerize to form humins,especially in aqueous systems and aqueous mixture systems,so initial fructose concentration has a large effect on the selectivity of HMF. We considered that in our system,the initial concentration of glucose also influenced the HMF yield. Table 1 shows the effects of initial glucose concentration,reaction temperature and time on glucose dehydration. When the glucose weight changed from 0.3 g to 0.9 g (Table 1,entries 1-4),the yield of HMF decreased from 78.4% to 71.7%,glucose conversion also decreased to 92.4%. Additional increase in glucose weight led to lower HMF selectivity and glucose conversion. This may have been due to the generation of humins in the aqueous-MIBK biphasic system,or that reactive compounds,such as glucose and HMF, would collide with each other or cross polymerize. Considering the cost of the reaction,we chose 0.7 g as the optimum initial glucose amount.
| Table 1 Effects of initial glucose concentration,reaction temperature and time (0.2 g IL-5, 1mL H2O,8mL MIBK,P = 2.5 atm.). |
As shown in Table 1,the reaction time and temperature played important roles both in glucose conversion and HMF yield (entries 5-14). With the elevation of temperature and prolonging of time, the conversion of glucose and HMF yield improved correspondingly. The glucose conversion was up to 99.7% with a HMF yield of 77.9% at 150 ℃ for 360 min. The HMF yield increased from 55.2% to 78.5% when reaction temperature changed from 80 ℃ to 140 ℃; as the reaction time increased from 180 min to 360 min,the glucose conversion changed from 87.5% to 97.4% at 120 ℃.
The color of the solution changed from colorless to deep brown as the reaction proceeded,which may be evidence for the decomposition of the formed HMF. From Table 1 we could see that when the temperature changed from 140 ℃ to 150 ℃,HMF yield decreased little and the yields of LA and HCOOH increased. Three side-reactions are possible. One is the rehydration of HMF into levulinic acid (LA) and formic acid; the second is the Aldol condensation between HMF molecules into soluble polymers; and the third is the cross-polymerization between HMF and glucose to form insoluble humins. The color of reacted solutions was brown; remaining even after HMF was extracted from the mixture. The brown by-products were thought to be soluble polymers and humins,which could not be quantified according to the present methods. In our work,the decrease in HMF selectivity was due to the side-reactions,which consumed the initial glucose and the formed HMF,and hence reduced the yield of HMF. 3.4. Effect of water content on glucose dehydration
Water is generally thought to have a negative effect on glucose dehydration into HMF [27, 28]. Since IL-5 is a hydrophilic ionic liquid,the influence of water content in the system on glucose dehydration was studied. As shown in Fig. 4,when the water content was 0.5 mL,the conversion of glucose was 95.6%,HMF and LA yield were up to 76.3% and 2.51%,respectively. However,as water content increased from 0.5 mL to 2.5 mL,HMF selectivity decreased significantly,while glucose conversion increased slightly. When H2O:MIBK was 1:8 (v/v),we got the optimum results. The conversion of glucose was 97.4% and HMF yield reached 75.1%. Fig. 4 also indicates that the yield of HMF decreased with the increase of water content. The reason was that when the dosage of water increased,the side effects of HMF rehydration happened,and the yield of by-product (LA) correspondingly increased. Therefore,water content in our system had a large influence on glucose dehydration,and also,water-MIBK biphasic system was an effective method for the reaction. Previous study [20] indicated that in pure water,the dehydration of fructose is generally nonselective; in our system,MIBK was added to extract continuously the HMF from the aqueous phase,so as to minimize degradation reactions arising from extended HMF residence in the reactive aqueous phase and to achieve more efficient recovery of HMF in the subsequent isolation step.

|
Download:
|
| Fig. 4.Influence of water content on the dehydration of glucose (0.7 g glucose,0.2 g IL-5,8 mL MIBK,T = 120 ℃,t = 360 min,P = 2.5 atm.). | |
To study the reusability of the catalyst IL-5,the main product HMF was separated from reaction mixture using extraction. After the first reaction run for glucose,only the catalyst IL-5 existed in the aqueous phase,so the organic phase was separated. Then 1 g of distilled water was added into the reaction liquid to decrease the viscosity of the ionic liquid and facilitate the extraction of HMF. Subsequently,the HMF was separated from the aqueous phase by extracting 8 times with 5 mL ethyl acetate [29]. The amount of extracted product was examined by HPLC,which demonstrated that ethyl acetate extraction could recover about 85%-90% of HMF. After extraction,the solution was heated at 60 ℃ in a vacuum drier until the water and residual ethyl acetate were removed absolutely. The dried IL-5 catalyst was used directly in the next run by adding fresh glucose and MIBK under the same reaction conditions. In order to examine the reusability of IL-5,the recycling tests were conducted five times. As shown in Fig. 5,the catalytic activity of the IL-5 for the dehydration of glucose did not decrease significantly after five runs,which demonstrated that the catalyst IL-5 was stable in this system. The non-complete extraction of HMF and LA and the residue of unreacted glucose in the previous run may have slight influence on the results of the recycling test. So, the separation and purification of HMF is a remaining subject of future study.

|
Download:
|
| Fig. 5.Recycling of IL-5 catalyst (0.7 g glucose,0.2 g IL-5,1 mL H2O,8 mL MIBK, T = 120 ℃,t = 360 min,P = 2.5 atm.). | |
In summary,we demonstrated that SO3H-functionalized acidic ionic liquids were efficient and excellent catalysts for the dehydration of glucose into 5-hydroxymethylfurfural (HMF). The acidity of the solution had a large effect on glucose conversion and HMF yield; the results showed that stronger acidity led to higher catalytic activity. The dosage of the catalyst and the initialamount of glucose also played important roles in the reaction. With the excessive elevation of temperature and prolonging of reaction time, the selectivity ofHMF decreased,which suggested the occurrence of the side reactions,such as rehydration,condensation,and reversion ofHMF. Glucose conversion reached 99.3% andHMFyieldwas 78.5% when the reactionwas performed at 140 ℃ for 360 min. The catalyst IL-5 was recycled,which exhibited favorable catalytic activity over five repeated runs. The simple,efficient,low toxicity,and reusable catalytic system has great potential for application.
AcknowledgmentsWe gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21276149), Shandong National Natural Science Funds (No. ZR2013BQ014) and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
| [1] | G. Yong, Y.G. Zhang, J.Y. Ying, Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose, Angew. Chem. Int. Ed. 47 (2008) 9345-9348. |
| [2] | F. Benvenuti, C. Carlini, P. Patrono, et al., Heterogeneous zirconium and titanium catalysts for the selective synthesis of 5-hydroxymethyl-2-furaldehyde from carbohydrates, Appl. Catal. A: Gen. 193 (2000) 147-153. |
| [3] | A.A. Rosatella, S.P. Simeonov, C.A.M. Afonso, et al., Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural, Green Chem. 13 (2011) 754-793. |
| [4] | F.M.A. Geilen, J. Klankermayer, W. Leitner, et al., Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system, Angew. Chem. Int. Ed. 49 (2010) 5510-5514. |
| [5] | K.I. Shimizu, R. Uozumi, A. Satsuma, Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods, Catal. Commun. 10 (2009) 1849-1853. |
| [6] | J.Y.G. Chan, Y.G. Zhang, Phosphotungstic acid encapsulated in metal-organic framework as catalysts for carbohydrate dehydration to 5-hydroxymethylfurfural, Chemsuschem 2 (2009) 731-734. |
| [7] | H.P. Yan, Y. Yang, D.M. Tong, et al., Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO4-2/ZrO2 and SO4-2/ZrO2-Al2O3 solid acid catalysts, Catal. Commun. 10 (2009) 1558-1563. |
| [8] | Y.M. Zhang, V. Degirmenci, C. Li, E.J.M. Hensen, Phosphotungstic acid encapsulated in metal-organic framework as catalysts for carbohydrate dehydration to 5- hydroxymethylfurfural, Chemsuschem 4 (2011) 59-64. |
| [9] | Z.H. Zhang, Z.K. Zhao, Production of 5-hydroxymethylfurfural from glucose catalyzed by hydroxyapatite supported chromium chloride, Bioresour. Technol. 102 (2011) 3970-3972. |
| [10] | R.L. Huang, W. Qi, R.X. Su, et al., Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural, Chem. Commun. 46 (2010) 1115- 1117. |
| [11] | M. Bicker, J. Hirth, H. Vogel, Dehydration of fructose to 5-hydroxymethylfurfural in sub and supercritical acetone, Green Chem. 5 (2003) 280-284. |
| [12] | M. Watanable, Y. Aizawa, T. Iida, et al., Glucose reactions with acid and base catalysts in hot compressed water at 473 K, Carbohydr. Res. 340 (2005) 1925- 1930. |
| [13] | Y. Nakamura, S. Morikawa, The dehydration of D-fructose to 5-hydroxymethyl-2- furaldehyde, Bull. Chem. Soc. Jpn. 53 (1980) 3705-3706. |
| [14] | K.I. Seri, Y. Inoue, H. Ishida, Highly efficient catalytic activity of lanthanide(Ⅲ) ions for conversion of saccharides to 5-hydroxymethyl-2-furfural in organic solvents, Chem. Lett. 29 (2000) 22-23. |
| [15] | K. Seri, Y. Inoue, H. Ishida, Catalytic activity of lanthanide(Ⅲ) ions for the dehydration of hexose to 5-hydroxymethyl-2-furaldehyde in water, Bull. Chem. Soc. Jpn. 74 (2001) 1145-1150. |
| [16] | F.S. Asghari, H. Yoshida, Acid-catalyzed production of 5-hydroxymethyl furfural from d-fructose in subcritical water, Ind. Eng. Chem. Res. 45 (2006) 2163-2173. |
| [17] | R.P. Swatloski, S.K. Spear, Y. Aizawa, R.D. Rogers, A novel cellulose hydrogel prepared from its ionic liquid solution, J. Am. Chem. Soc. 124 (2002) 4974-4975. |
| [18] | X. Tong, Y. Ma, Y. Li, An efficient catalytic dehydration of fructose and sucrose to 5- hydroxymethylfurfural with protic ionic liquids, Cabohydr. Res. 345 (2010) 1698- 1701. |
| [19] | H. Zhao, J.E. Holladay, H. Brown, Z.C. Zhang, Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural, Science 316 (2007) 1597- 1599. |
| [20] | M. Lu, X.H. Guan, X.H. Xu, D.Z. Wei, Characteristic and mechanism of Cr(VI) adsorption by ammonium sulfamate-bacterial cellulose in aqueous solutions, Chin. Chem. Lett. 24 (2013) 253-256. |
| [21] | Y. Román-Leshkov, J.N. Chheda, J.A. Dumesic, Phase modifiers promote efficient production of hydroxymethylfurfural from fructose, Science 312 (2006) 1933- 1937. |
| [22] | F. Tao, H. Song, L. Chou, Efficient process for the conversion of xylose to furfural with acidic ionic liquid, Can. J. Chem. 89 (2011) 83-87. |
| [23] | K. Niknam, M. Damya, 1-Butyl-3-methylimidazolium hydrogen sulfate[bmim]HSO4: an efficient reusable acidic ionic liquid for the synthesis of 1,8- dioxo-octahydroxanthenes, J. Chin. Chem. Soc. 56 (2009) 659-665. |
| [24] | A.C. Cole, J.L. Jensen, L. Ntai, et al., Novel Brønsted acidic ionic liquids and their use as dual solvent-catalysts, J. Am. Chem. Soc. 124 (2002) 5962-5963. |
| [25] | Q. Zhao, L. Wang, S. Zhao, et al., High selective production of 5-hydroymethylfurfural from fructose by a solid heteropolyacid catalyst, Fuel 90 (2011) 2289- 2293. |
| [26] | B.F.M. Kuster, 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture, Starch 42 (1990) 314-321. |
| [27] | X.H. Qi, M. Watanabe, R.L. Smith, et al., Efficient process for conversion of fructose to 5-hydroxymethylfurfural with ionic liquids, Green Chem. 11 (2009) 1327- 1331. |
| [28] | S.Q. Hu, Z.F. Zhang, Y.X. Zhou, et al., Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials, Green Chem. 10 (2008) 1280-1283. |
| [29] | X.H. Qi, M. Watanabe, T.M. Aida, et al., Efficient conversion of glucose into 5- hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid, Green Chem. 11 (2009) 1327-1331. |