b College of Environmental Science and Engineering, Nankai University, Tianjin 300071, China
1. Introduction
Triazines have been widely used in agricultural domain due to their high power for weed control for many years. The wide use of these herbicides at large scale has resulted in serious pollution of the environment [1]. Triazine herbicides and their degradation products are highly toxic,mobile and soluble in water and can also be strongly adsorbed onto soil,hence have been an environmental safety concern [2, 3]. In the United States,a joint project of the Environmental Protection Agency’s Office of Drinking Water (ODW) and the Office of Pesticide Programs carried out a national pesticide survey [4, 5],and the results showed that atrazine and simazine existed in the drinking water samples. In the EU,the maximum allowed limit for each individual herbicide has been set at 0.1 mg/L [6]. Hence it is necessary to develop simple,sensitive and low cost monitoring techniques for the analysis of the residual level of these pollutants.
Generally,a sample pretreatment step is used before an effective method is developed,and up to now,many methods have been established for the enrichment of triazines residues from complex matrices. Liquid-liquid extraction often needed a large volume of the sample of interest and toxic organic solvents and also was labor intensive,expensive,and time-consuming. Singledrop microextraction is established based on the principle of liquid-liquid extraction and the enrichment is finished in a single drop of solvent. However,the shortcomings of this method are the instability of the drop when fast stirring or volatile organic solvents are used [7]. This method is also time-consuming and the equilibrium requires a long time to achieve in most cases. Dispersive liquid-liquid microextraction [8] is a recently constructed method and has many applications for the determination of pesticides,pharmaceuticals,PCBs,PBDEs and PAHs,etc. However,chlorobenzene,chloroform and carbon disulfide were often used as the extraction solvent and methanol,acetonitrile and acetone etc. were used as the disperser solvents,which will result in secondary pollution. In order to resolve such problems,ionic liquid (IL) was an ideal selection because ionic liquids have many interesting properties including wide liquid ranges,low volatility, good stability,reusability,etc. Due to these useful properties,ILs have been widely used in organic chemistry and analytical chemistry. In dispersive liquid-liquid microextraction processes, ILs also have some applications for the analysis of hexabromocyclododecane diastereomers [9],polar herbicides [10],pesticides and heavy metals,etc. [11, 12, 13]. In this work,1-octyl-3-methylimidazolium hexafluorophosphate [C8MIM][PF6] was chosen as the extraction solvent and triazine herbicides were selected as the model analytes. A new temperature controlled ionic liquid dispersive liquid phase microextraction was developed for the sensitive determination of triazine herbicides. The possible factors were optimized.
2. Experimental
Cyanazine,simazine,and atrazine were purchased from AccuStandard,Inc. (New Haven,USA). The stock solutions with a concentration of 1000 mg/L were prepared by dissolving the solid bulk triazine herbicides in methanol and were stored at 4 ℃ in a refrigerator. The working solutions were obtained by diluting the stock solutions with methanol. 1-Octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]) was synthesized in our own laboratory [14]. HPLC grade methanol and acetonitrile were purchased from Jiangsu Guoda Chemical Reagent Co.,Ltd. (Huaian, China). Ultrapure water used in the experiments was prepared on a Milli-Q water purification system (Millipore,Bedford,MA,USA). All glass used in the experiments was washed with pure water,then soaked in 6 mol/L nitric acid for 24 h and cleaned with ultrapure water before use.
The analysis and separation was carried out on a high performance liquid chromatography system including two LC-10 ATvp pumps and an SPD-10 Avp ultraviolet detector (Shimadzu, Kyoto,Japan). The analytical column was a reversed-phase SunFire C18 column (150 mm × 4.6 mm,particle size 5 mm) and the software of Chromato Solution Light Chemstation for LC system was used to acquire and process chromatographic data. The mobile phase was a mixture of methanol and ultrapure water (40/60,v/v). The mobile phase flow rate was set at 1.2 mL/min,the injection volume was set at 20 mL,and the detection wavelength was set at 275 nm. An Anke 80-2 (Shanghai,China) centrifuge was utilized to centrifuge the cloudy water solutions.
The enrichment procedure was as follows: 10 mL of ultra-pure water was put in a 10 mL conical tube,the cyanazine,simazine and atrazine were spiked with a concentration of 10 mg/L,20 mg/L and 20 mg/L,respectively. Then sodium chloride was added at a concentration of 20% (w/v). After 50 mL of [C8MIM][PF6] was added,the tube was heated in a water bath with the temperature controlled at 80 ℃. When [C8MIM][PF6] was completely dissolved in the aqueous solution and a nearly homogenous solution was obtained,the tube was cooled with ice water. The tube was kept for 30 min to enhance the extraction of triazine herbicides from the sample solution into the tiny droplets of [C8MIM][PF6]. Thereafter the mixture was centrifuged for 20 min at 4000 rpm. The upper aqueous phase was removed,and the residue was dissolved in 200 mL of methanol and 20 mL of the resulting solution was injected into the HPLC system for analysis. Each experiment was done in triplicate to ensure the accuracy of the experimental data.
Four water samples were collected for use. River water was taken from Baihe,Nanyang City,Henan Province,China. Underground water was obtained from Henan Normal University in Xinxiang City,Henan Province,China. Drainage water was collected from Gongchanzhuyi drainage,Xinxiang City,Henan Province,China. Wastewater was obtained from the outlet of Luotuowan sewage treatment plant,Xinxiang City,Henan Province,China. Before use,all the environmental water samples were filtered with 0.45 mm micro-pore membranes and then stored in brown glass containers at a low temperature of 4 ℃.
3. Results and discussion 3.1. Effect of ionic strength
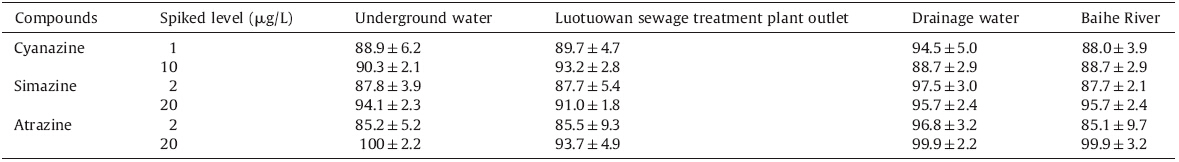
The salting-out effect is often present in many cases,and it is often utilized to enhance the enrichment or preconcentration performance. In theory,addition of salt will decrease the solubility of analytes in the aqueous sample and enhances their partitioning into the adsorbent or organic phase. On the other hand,the addition of salt increased the solubility of the ionic liquid in water, which played a dominant role in the decrease of the volume of the sediment phase. A series of experiments were designed to investigate the salting-out effect (Fig. 1). The results demonstrated that a significant increase of the peak area occurred as the ionic strength of the solution increased from 0% to 20% (w/v),but the peak area decreased when the ionic strength of the solution was further increased to the range of 20%-25%. So 20% (w/v) NaCl was added in the following experiments.
|
Download:
|
| Fig. 1.Effect of sodium chloride content. Conditions: [C8MIM][PF6] volume,50 mL; sample volume,10 mL; spiked concentration,10 mg/L for cyanazine,20 mg/L for simazine and atrazine; temperature,70 ℃; extraction time,30 min; centrifuging time,20 min; (■) cyanazine,(●) simazine,(▲) atrazine. | |
3.2. Effect of the volume of ionic liquid
The volume of extraction solvent was a key factor in the newly developed procedure,which determines the enrichment performance to a certain extent. In order to achieve the maximum enrichment performance possible,the volume of extraction solvent was optimized in the range of 40-60 mL (Fig. 2). From Fig. 2,we can see that the largest peak area of the targeted analytes was obtained when the volume of extraction solvent was 50 mL. The peak area increased with the increase of IL volume in the range of 40-50 mL. Even larger volume of IL led to the decrease of the peak area when the volume of IL was increased in the range of 50- 60 mL. The reason may be that the superfluous IL could not be dispersed into the solution as microdrops and absorb onto the wall of the tube,and some analytes migrated into this part of IL. So this part of the analytes would not be sedimented into the bottom of the tube,leading to the decrease of the peak area. Hence,50 mL of [C8MIM][PF6] was chosen in the subsequent experiments.

|
Download:
|
| Fig. 2. Effect of ionic liquid volume. Conditions: ionic liquid,[C8MIM][PF6]; sample volume,10 mL; spiked concentration,10 mg/L for cyanazine,20 mg/L for simazine and atrazine; sodium chloride content,20% (w/v); temperature,70 8C; extraction time,30 min; centrifuging time,20 min. (■) cyanazine,(●) simazine,(▲) atrazine. | |
3.3. Effect of dissolving temperature
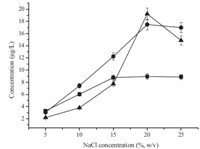
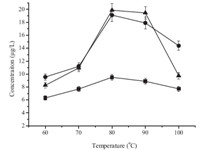
In this newly established process,temperature is a crucial factor because it is the driving force for the complete dispersion of [C8MIM][PF6] into the aqueous solution. In order to achieve the best temperature for extraction,temperature was investigated in the range of 60-100 ℃ (Fig. 3). FromFig. 3,we can see that 80 ℃was the reasonable temperature because the largest peak area occurred at 80 ℃. The probable reasons are that the complete dispersion extent of [C8MIM][PF6] into the aqueous solution increased along with the rise of temperature and the migrating-in speed was equal to the migrating-out speed into the ionic liquid phase. However the recoveries of analytes obtained over 80 ℃ was lower than those obtained at 80 ℃.Maybe the migrating-in and migrating-out speeds increased at the same time,but the migrating-out speed was larger than the migrating-in speed to the ionic liquidphase. This resulted in a decreased distribution of analytes in the ionic liquid phase. So the dissolving temperature was set at 80 ℃.
|
Download:
|
| Fig. 3. Effect of temperature. [C8MIM][PF6] volume,50 mL; sample volume,10 mL; spiked concentration,10 mg/L for cyanazine,20 mg/L for simazine and atrazine; sodium chloride content,20% (w/v); extraction time,30 min; centrifuging time, 20 min. (■) cyanazine,(●) simazine,(▲) atrazine. | |
3.4. Sample pH value
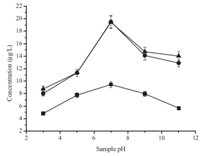
The sample pH value is also a key factor in the sample pretreatment techniques,which determines the present form of the analytes and then affects extraction efficiency. Therefore,the effect of sample pH value has to be investigated. In this study,it was carried out in the range of pH 3-11 (Fig. 4). The peak areas increased with the increase of pH value in the range of pH 3-7,and decreased in the range of pH 7-11. This phenomenon was closely related to their chemical properties. Atrazine and simazine can be hydrolyzed in strong acid or alkali environment at high temperature. Based on these results,sample pH value was set at 7 in all subsequent experiments.
|
Download:
|
| Fig. 4. Effect of sample pH. Conditions: ionic liquid,[C8MIM][PF6]; sample volume, 10 mL; spiked concentration,10 mg/L for cyanazine,20 mg/L for simazine and atrazine; sodium chloride content,20% (w/v); temperature,80 8C; extraction time, 30 min; centrifuging time,20 min. (■) cyanazine,(●) simazine,(▲) atrazine. | |
3.5. Effect of extraction time and centrifuging time
Mass transfer is a time dependent process and one of the salient factors in most extraction procedures,especially inmicroextraction methods such as SPME and LPME. In this procedure,the mass transfer between the targeted compounds and ionic liquid phase will be related to various factors and reach an equilibriumin the end. The effect of extraction time was examined in the range of 10- 50 min. The experimental results showed that the best extraction performancewas achieved at 30min. Centrifugation is a critical step in order to obtain two distinguishable phases,so it will affects the size of the settled phase and the concentration of analytes in the extraction phase. The centrifugation time was checked in the range of 5-25 min at 4000 rpmafter extraction. The results indicated that the peak areas of the analytes increased as the centrifugation time increased from 5 min to 15 min,and decreased after 15 min. The sedimented volume increased along with the time,but longer centrifuging time resulted in heat generation,which led to the redissolution of some [C8MIM][PF6] into the water phase and a loss of extraction performance. Finally,15min was used.
3.6. Analytical performance and real water sample analysis
To evaluate the proposed method,often-used parameters such as linear range,precisions and detection limits were investigated under the optimal experimental conditions (Table 1). The linear range was in the range of 0.5-80 mg/L for cyanazine,and 1.0- 100 mg/L for simazine and atrazine,respectively. Good linearity with correlation coefficient (R2) in the range of 0.9939-0.9965 and low detection limit in the range of 0.05-0.06 mg/L based on three times of the ratio of signal to noise (S/N = 3) were achieved. The intra day precision and inter day precision was in the ranges of 3.2%-6.6% and 4.6%-8.9% (RSD,n = 6),respectively.
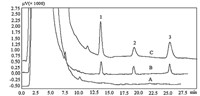
To demonstrate the feasibility of the proposed method,it was used to determine the triazine herbicides in four real water samples (Table 2). The targeted analytes were not found in the blank samples of the four samples. These samples were spiked with two different concentrations,1 mg/L for cyanazine,2 mg/L for simazine and atrazine,and 10 mg/L for cyanazine,20 mg/L for simazine and atrazine,respectively. As can be seen from Table 2, good spiked recoveries in the range of 85.1%-100% with the precisions of 1.8%-9.7% (RSD) were obtained. The typical chromatogram of water samples was showed [(Fig._3)TD$FIG] in Fig. 5.
| Table 1 Linear ranges,precisions,detection limits for the enrichment of triazine herbicides by temperature controlled ionic liquid dispersive liquid phase micro-extraction. |
| Table 2 Spiked recoveries in real water samples by the proposed method. |
|
Download:
|
| Fig. 5. Typical chromatogram of Baihe water sample. (A) Blank; (B) spiked concentration,1 mg/L for cyanazine,2 mg/L for simazine and atrazine; (C) spiked concentration, 10 mg/L for cyanazine,20 mg/L for simazine and atrazine. Peak identification,(1) cyanazine,(2) simazine,(3) atrazine. | |
4. Conclusion
This paper described a simple and sensitive temperaturecontrolled ionic liquid dispersive liquid phase microextraction in combination with HPLC for the analysis of triazine herbicides in environmental water samples. This developed extraction system is a cheap,convenient sample pretreatment technique,and reduces the exposure danger to the toxic solvents used in the conventional extraction procedure. Under the best extraction conditions,low limit of detection,good linearity and repeatability were achieved. The analysis of real water samples indicated that the method was excellent and would be very competitive in the routine analysis of environment pollutants.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21377167),Program for New Century Excellent Talents in University (No. NCET-10-0813).
| [1] | R.A. Boyd, Herbicides and herbicide degradates in shallow groundwater and the Cedar River near a municipal well field, Cedar Rapids, Iowa, Sci. Total Environ. 248 (2000) 241-253. |
| [2] | G. Shen, H.K. Lee, Determination of triazines in soil by microwave-assisted extraction followed by solid-phase microextraction and gas chromatography- mass spectrometry, J. Chromatogr. A 985 (2003) 167-174. |
| [3] | Y. Wang, J.Y. You, R.B. Ren, et al., Determination of triazines in honey by dispersive liquid-liquid microextraction high-performance liquid chromatography, J. Chromatogr. A 1217 (2010) 4241-4246. |
| [4] | Drinking Water Inspectorate, A report by the Chief Inspector, Drinking Water 1993, HMSO, London, 1994. |
| [5] | US Environmental Protection Agency, National Survey of Pesticides in Drinking Water Wells, Phase I Report, EPA PB-91-125765, National Technical Information Services, Springfield, VA, 1990. |
| [6] | EC Drinking Water Guideline, 98/83/CE, Brussels, November (1998). |
| [7] | C.L. Ye, Q.X. Zhou, X.M. Wang, Improved single-drop microextraction for high sensitive analysis, J. Chromatogr. A 1139 (2007) 7-13. |
| [8] | M. Rezaee, Y. Yamini, M. Faraji, Evolution of dispersive liquid-liquid microextraction method, J. Chromatogr. A 1217 (2010) 2342-2357. |
| [9] | R.S. Zhao, X. Wang, J.P. Yuan, S.S. Wang, C.G. Chen, Trace determination of hexabromocyclododecane diastereomers in water samples with temperature controlled ionic liquid dispersive liquid phase microextraction, Chin. Chem. Lett. 22 (2011) 883-886. |
| [10] | S. Wang, L. Ren, C.Y. Liu, J. Ge, F.M. Liu, Determination of five polar herbicides in water samples by ionic liquid dispersive liquid-phase microextraction, Anal. Bioanal. Chem. 397 (2010) 3089-3095. |
| [11] | Q.X. Zhou, H.H. Bai, G.H. Xie, J.P. Xiao, Temperature-controlled ionic liquid dispersive liquid phase micro-extraction, J. Chromatogr. A 1177 (2008) 43-49. |
| [12] | Q.X. Zhou, H.H. Bai, G.H. Xie, J.P. Xiao, Trace determination of organophosphorus pesticides in environmental samples by temperature-controlled ionic liquid dispersive liquid-phase microextraction, J. Chromatogr. A 1188 (2008) 148-153. |
| [13] | H.H. Bai, Q.X. Zhou, G.H. Xie, J.P. Xiao, Temperature-controlled ionic liquid-liquidphase microextraction for the pre-concentration of lead from environmental samples prior to flame atomic absorption spectrometry, Talanta 80 (2010) 1638-1642. |
| [14] | G. Cravotto, L. Boffa, J.M. Lévêque, et al., A speedy one-pot synthesis of secondgeneration ionic liquids under ultrasound and/or microwave, Aust. J. Chem. 60 (2007) 946-950. |