b Chemistry Departments, V. P. and R. P. T. P Science College, Affiliated to Sardar Patel University, Vallabh Vidyanagar 388120, India
1. Introduction
The discovery of new biologically active molecules with high potency represents a high priority for medicinal chemists because the common pathogenic species have developed drug resistance against available anti-infectious medicines [1]. To address this emerging challenge,we have attempted to synthesize different types of biologically active compounds,particularly biologically active quinazolin-4(3H)-one derivatives in recent years [2, 3, 4]. The quinazolin-4(3H)-one moiety is associated with promising activities such as hypnotic,sedative,analgesic,anti-convulsant,antitussive, anti-bacterial,anti-diabetic,anti-inflammatory effects and many more [5, 6, 7]. In addition to that,researchers have been interested in designing compounds bearing pyrazole heterocycles in the backbone,due to their diverse biological utilities such as antimicrobial [8],anti-inflammatory [9],antioxidant [10],antitumor [11],anti-TB [12],analgesic and ulcerogenic activities [13].
a-Amino acids are amphoteric in nature and are found as zwitter ions,are useful in various organic transformations like coupling reactions,Mannich type addition reactions,aldol condensations and many asymmetric syntheses. L-Proline is one of the amino acids that have very significant roles in catalysis [14, 15, 16, 17, 18, 19].
From the perusal of above literature survey,we undertook the synthesis of structurally hybrid molecules containing both quinazolin-4(3H)-one and pyrazole scaffolds in a single molecular framework using L-proline as a catalyst,which may improve the pharmacological activity of the synthesized compounds. Structural characterization of prepared compounds has been carried out using FT-IR,mass,1H NMR and 13C NMR spectroscopy. In vitro antimicrobial study for all the synthesized compounds was carried out against six bacterial and two fungal species using the broth dilution MIC (minimum inhibitory concentration) method [20]. Antituberculosis activity was carried out utilizing the in vitro mycobacterial susceptibility tests [21].
2. Experimental
All the chemicals used for syntheses and biological assays were obtained from local vendors (make-Merck India,Pvt. Ltd.) and used without further purification. The progress of the reaction was monitored by TLC analysis on aluminum sheets coated with silica get 60 F254 (Merck) using hexane:EtOAc:MeOH = 8:2:0.5 solvent system as the mobile phase by UV visualization. Melting point (uncorrected) for all the synthesized compounds was assessed using a digital melting point apparatus. Elemental analysis for each compound was carried out using a CARLO ERBA-1108 elemental analyzer. Mass spectral analysis was carried out using a Thermo fisher Scientific,MS-LCQ Fleet instrument. The FT-IR spectral analysis was performed using a SHIMADZU 8400s spectroscopic grade KBr pellets in a frequency range of 400-4000 cm-1. The 1H NMR and 13C NMR spectra were recorded on a Bruker 400 MHz instrument using DMSO as a solvent as well as an internal reference standard.
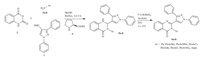
General procedure for the synthesis of compounds 5a-h and 6a-h: Synthesis of the titled compounds 5a-h was carried out using 1-phenyl-3-aryl-1H-pyrazole-4-carbaldehyde 1 (0.002 mol), isatioc anhydride 2 (0.002 mol),aromatic amines 3a-h (0.002 mol) and L-proline 4 (5 mol%) in 10 mL of MeOH in a 25 mL round bottom flask attached with a reflux condenser. Reaction mixture was refluxed for 1-2.5 h and the precipitates were filtered and recrystallized from MeOH. Compounds 5a-h were oxidized using 5% KMnO4 in acetone for 8 h at room temperature and the product was extracted with chloroform. Excess of chloroform was removed in vacuo and the crystals of product 6a-h were separated.
Physical data,spectroscopic characterizations and experimental protocols for antimicrobial and antituberculosis for compounds 5a-h and 6a-h are given in Supporting information.
3. Results and discussion 3.1. Chemistry
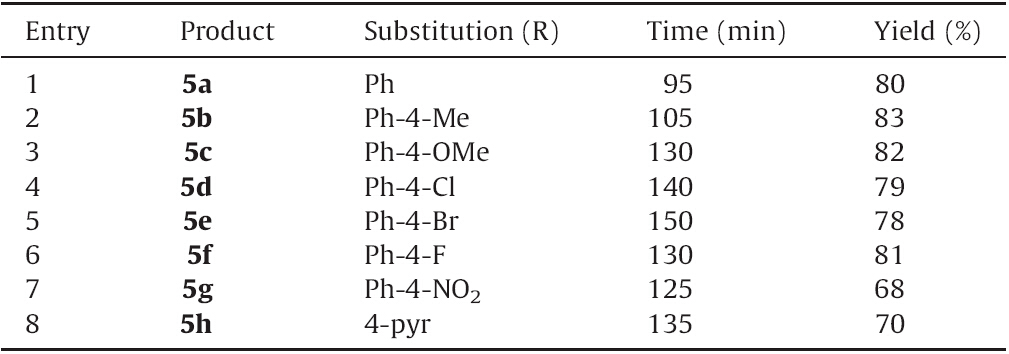
The synthesis route of the titled compounds (5a-h and 6a-h) is shown in Scheme 1. The first intermediate required for synthesis of the titled compounds,1-phenyl-3-aryl-1H-pyrazole-4-carbaldehyde 1,was prepared using a literature method [22]. It was made by the Vilsmeier-Haack formylation of hydrazone prepared from acetophenone and phenyl hydrazine. Compounds 5a-h were prepared using a one-pot multi-component reaction between 1- phenyl-3-aryl-1H-pyrazole-4-carbaldehyde 1,isatioc anhydride 2 and aromatic amines 3a-h using L-proline as a catalyst in methanol,which was further oxidized using 5% neutral KMnO4 in acetone to produce final products 6a-h. The prepared compounds were obtained in comparatively good yields (68%- 91%). A possible mechanism for the reaction is shown in Scheme 2. The first step of the reaction is the formation of an enamine,which provides a highly electrophilic carbon center for the nucleophilic nitrogen of amine to form the intermediate (X). Intermediate (X) undergoes subsequent steps such as decarboxylation,attack of aldehyde,formation of aldimine,cyclization and finally elimination to give final products 5a-h,which were further oxidized to produce 6a-h. Different concentrations of catalyst were studied and the optimum loading appeared to be 5 mol% as shown in Table 1. Time required and yields obtained for all the titled compounds 5a-h are shown in Table 2.
|
Download:
|
| Scheme 1.Synthetic route for entitled compounds 5a–h and 6a–h. | |

|
Download:
|
| Scheme 2.Plausible reaction mechanism for the role of L-proline as a catalyst. | |
| Table 1 Comparison of the time required and yield obtained under different catalytic condition for the synthesis of 5a. |
| Table 2 Time required and yield obtained for entitled compounds 5a–h. |
Titled compounds 5a-h and 6a-h were characterized using FTIR, mass,1H NMR and 13C NMR spectroscopic methods. FT-IR spectra of compounds showed characteristic bands for functionalities such as -NH,C55O,C-N-C linkage of quinazolinone,F,NO2,Cl and Br around 3220,1700,1591/1540,1225,1530/1350,750 and 620 cm-1 respectively. In addition,band due to -NH stretching in compounds 5a-h around 3220 cm-1 disappeared in compounds 6a-h upon oxidation. In 1H NMR spectra of the titled compounds, aliphatic protons of -CH3,OCH3 and CHNH appeared at d 2.17- 2.19,3.89-3.90 and d 6.40-6.51 in compounds 5b/6b,5c/6c and 5a-h respectively. All the aromatic protons present in quinazolinone and phenyl rings appeared around d 7.05-8.10. Furthermore, protons of -NH in the quinazolinone ring and CH of pyrazole ring appeared at around d 8.15 and 8.70 as a doublet and a singlet respectively. In 13CNMRaliphatic carbons appeared at around d 24, 53 and 66 due to the presence of -CH3,OCH3 and CHNH in compounds 5b/6b,5c/6c and 5a-h respectively. Aromatic carbons present in the structure,gave peaks in the range of d 114-157. Moreover,carbons of C-OCH3 and C55O appeared at d 156 and 163/ 161 in 5c/6c and 5a-h/6a-h respectively. Results of elemental analysis of all the synthesized compounds are in good agreement with the calculated data. In addition,mass spectra of all the compounds showed molecular ion M+ (two peaks in case of chloro and bromo derivatives) corresponding to their exact mass.
3.2. Biology
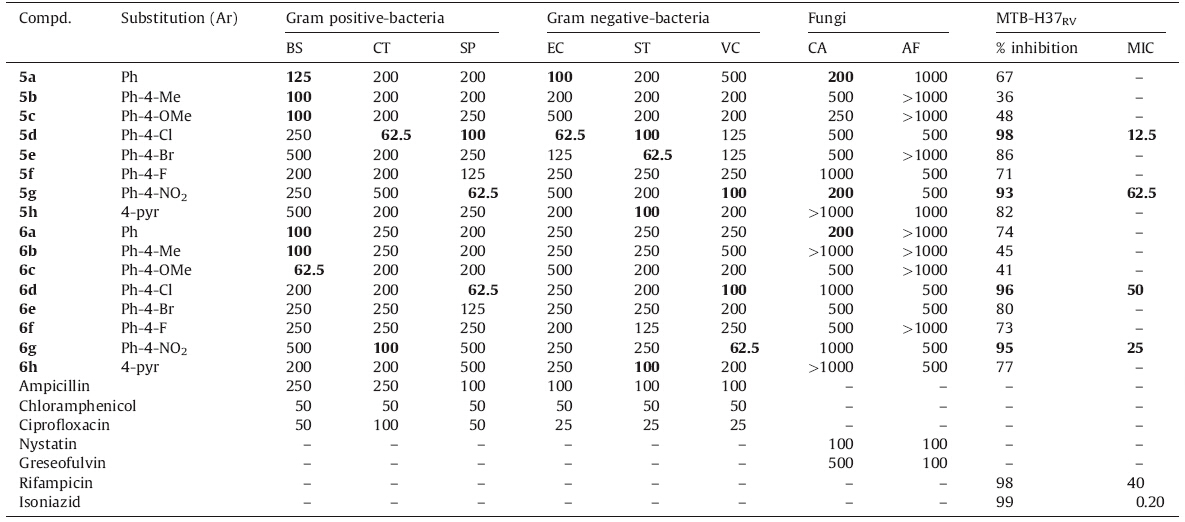
In case of antimicrobial screening (Table 3) for gram positive bacteria,compound 6c (62.5 mg/mL) showed superior whereas 5b, 5c,6a and 6b (100 mg/mL) showed equivalent activity against Bacillus subtilis as compared to Ampicillin. Against Clostridium tetani,compound 5d (62.5 mg/mL) exhibited significant inhibition while 6g showed comparable inhibition (100 mg/mL) to Ciprofloxacin. Amongst all tested compounds 5g and 6d showed good activity (62.5 mg/mL) while 5d showed similar activity (100 mg/ mL) against Streptococcus pnumonia when compared with Ampicillin. In case of gram negative bacteria,compound 5d (62.5 mg/ mL) found to possess excellent inhibition while compound 5a (100 mg/mL) demonstrated similar activity against Escherichia coli compared to Ampicillin. Compounds 5e and 6g (62.5 mg/mL) showed significant potency whereas 5d,5h,6h and 5g,6d (100 mg/ mL) showed similar activity against Salmonella typhi,Vibrio cholerae as compared to Ampicilin.
| Table 3 In vitro antibacterial, antifungal and antimycobacterial activity (MIC, mg/mL) of compounds 5a–h and 6a–h. |
The anti fungal (Table 3) activity results revealed that amongst all the tested compounds 5a,6a and 5g (200 mg/mL) showed superior activity against Candida albicans compared to the standard drug Greseofulvin but none of the compounds displayed higher potency against Aspergillus fumigates. Rest of the compounds exhibited good to moderate activity against tested organisms but not as effective as the standard drugs.
Structural activity relationship (SAR) study revealed that change of substitutions or replacement of one of the carbon atom with nitrogen in phenyl ring present at the 3-position of quinazolinone ring may enhance or diminish the potency. Introduction of electron donating groups (-CH3 and -OCH3) in parent structure improved the strength against gram positive bacteria B. subtilis but retained or lost potency in case of other bacterial species. On the other hand,electron withdrawing groups (-Cl,-Br and -NO2) may improve the activity up to 3-4-fold against bacterial species. In case of antifungal activity,alteration of substitution at the phenyl ring decreased the activity of the titled compounds.
Antituberculosis (Table 3) activity results revealed that only compounds 5d,5g,6d and 6g showed >90% of inhibition using Rifampicin and Isoniazid as positive controls against Mycobacterium tuberculosis H37RV bacteria. SAR analysis demonstrated that compounds with -Cl and -NO2 groups showed significant improvement in % inhibition in comparison with the unsubstituted compound. Compounds 5d and 6g showed considerably higher MIC values than Rifampicin.
4. Conclusion
The synthetic strategy disclosed here permits the assembly of two biologically active motifs into one heterocyclic framework,i.e. pyrazolyl quinazolin-4(3H)-one derivatives. From SAR study,we conclude that substituents at the phenyl ring adjacent to the pyrazole ring have significant effects on the potency.
Acknowledgments
The authors are thankful to Director,Ashok and Rita Patel Institute of Integrated Study and Research in Biotechnology and Allied Sciences,Chairman,Charutar Vidya Mandal & Director, SICART for providing all necessary research facilities.
Appendix A. Supplementary data
Supplementarymaterial relatedto this article canbe found,inthe online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.015.
| [1] | V. Aloush, S. Navon-Venezia, Y. Seigman-Igra, S. Cabili, Y. Carmeli, Multidrugresistant Pseudomonas aeruginosa: risk factors and clinical impact, Antimicrob. Agents Chemother. 50 (2006) 43-48. |
| [2] | R.B. Dixit, A.P. Bulsara, H.B. Mehta, B.C. Dixit, Synthesis, characterization and antimicrobial activities of new bis-hydrazonothioxothiazolidinone derivatives, J. Saudi Chem. Soc. 16 (2012) 193-197. |
| [3] | R.B. Dixit, S.F. Vanparia, T.S. Patel, et al., Synthesis and antimicrobial activities of sulfonohydrazide-substituted 8-hydroxyquinoline derivative and its oxinates, Appl. Organomet. Chem. 24 (2010) 408-413. |
| [4] | R.B. Dixit, T.S. Patel, S.F. Vanparia, et al., DNA-binding interaction studies of microwave assisted synthesized sulfonamide substituted 8-hydroxyquinoline derivatives, Sci. Pharm. 79 (2011) 293-308. |
| [5] | C.L. Jagani, N.A. Sojitra, S.F. Vanparia, et al., Microwave promoted synthesis and antimicrobial activity of 3-thiazole substituted 2-styryl-4(3H)-quinazolinone derivatives, J. Saudi Chem. Soc. 16 (2012) 363-369. |
| [6] | N.A. Sojitra, R.B. Dixit, R.K. Patel, J.P. Patel, B.C. Dixit, Classical and microwave assisted synthesis of new 4-(3,5-dimethyl-1-phenyl-1H-pyrazol-4-ylazo)-N-(2- substituted-4-oxo-4H-quinazolin-3-yl)benzenesulfonamide derivatives and their antimicrobial activities, J. Saudi Chem. Soc. (2012), http://dx.doi.org/ 10.1016/j.jscs.2012.07.020. |
| [7] | (a) D.A. Patil, P.O. Patil, G.B. Patil, S.J. Surana, Synthesis of 2,3-disubstitutedquinazolin- 4-(3H)-ones, Mini Rev. Med. Chem. 11 (2011) 633-641; (b) H.H. Jardosh, M.P. Patel, Lanthanum triflate-triggered synthesis of tetrahydroquinazolinone derivatives of N-allyl quinolone and their biological assessment, J. Serb. Chem. Soc. 77 (2012) 1561-1570; (c) R.S. Giri, H.M. Thaker, T. Giordano, et al., Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-kB and AP-1 mediated transcription activation and as potential anti-inflammatory agents, Eur. J. Med. Chem. 44 (2009) 2184- 2189. |
| [8] | H.H. Jardosh, C.B. Sangani, M.P. Patel, R.G. Patel, One step synthesis of pyrido[1,2- a]benzimidazole derivatives of aryloxypyrazole and their antimicrobial evaluation, Chin. Chem. Lett. 24 (2013) 123-126. |
| [9] | B.V. Kendre, M.G. Landge, W.N. Jadhav, S.R. Bhusare, Synthesis and bioactivities of some new 1H-pyrazole derivatives containing an aryl sulfonate moiety, Chin. Chem. Lett. 24 (2013) 325-328. |
| [10] | V. Sharath, H.V. Kumar, N. Naik, Synthesis of novel indole based scaffolds holding pyrazole ring as anti-inflammatory and antioxidant agents, J. Pharm. Res. 6 (2013) 785-790. |
| [11] | X.J. Song, Y. Shao, X.G. Dong, Microwave-assisted synthesis of some novel fluorinated pyrazolo[3,4-d]pyrimidine derivatives containing 1,3,4-thiadiazole as potential antitumor agents, Chin. Chem. Lett. 22 (2011) 1036-1038. |
| [12] | P. Aragade, M. Palkar, P. Ronad, D. Satyanarayana, Coumarinyl pyrazole derivatives of INH: promising antimycobacterial agents, Med. Chem. Res. 22 (2013) 2279-2283. |
| [13] | M. Amir, S. Kumar, Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of 3,5-dimethyl pyrazoles, 3-methyl pyrazol-5-ones and 3,5-disubstituted pyrazolines, Indian J. Chem. 44B (2005) 2532-2537. |
| [14] | F.K. Behbahani, H.S. Alaei, L-Proline-catalysed synthesis of functionalized unsymmetrical dihydro-1H-indeno[1,2-b]pyridines, J. Chem. Sci. 125 (2013) 623-626. |
| [15] | F.K. Behbahani, M. Ghorbani, M. Sadeghpour, M. Mirzaei, L-Proline as reusable and organo catalyst for the one-pot synthesis of substituted 2-amino-4H-chromenes, Lett. Org. Chem. 10 (2013) 191-194. |
| [16] | C.L. Shi, J.X. Wang, H. Chen, D.Q. Shi, Regioselective synthesis and in vitro anticancer activity of 4-aza-podophyllotoxin derivatives catalyzed by L-proline, J. Comb. Chem. 12 (2010) 430-434. |
| [17] | L.W. Xu, Z.T. Wang, C.G. Xia, L. Li, P.Q. Zhao, Improved protocol for the threecomponent Biginelli reactions and Biginelli-like Mannich reactions of carbamates, aldehydes and ketones, Helv. Chim. Acta 87 (2004) 2608-2612. |
| [18] | J.M. Khurana, B. Nand, S. Kumar, Rapid synthesis of polyfunctionalized pyrano[ 2,3-c]pyrazoles via multicomponent condensation at room-temperature ionic liquids, Synth. Commun. 41 (2011) 405-410. |
| [19] | X. Gao, H. Fu, R. Qiao, Y. Jiang, Y. Zhao, Copper-catalyzed synthesis of primary arylamines via Cascade reactions of aryl halides with amidine hydrochlorides, J. Org. Chem. 73 (2008) 6864-6866. |
| [20] | National Committee for Clinical Laboratory Standards (NCCLS), Performance Standards for Antimicrobial Susceptibility Testing; Twelfth Informational Supplement, Wayne, Pennsylvania, USA, 2002 M100-S12 M7. |
| [21] | R. Schwalbe, L. Steele-Moore, A. Goodwin, Antimicrobial Susceptibility Testing Protocols, CRC Press, Boca Raton, 2007. |
| [22] | A. Kira, M.O. Abdel-Raeman, K.Z. Gadalla, The Vilsmeier-Haack reaction-Ⅲ cyclization of hydrazones to pyrazoles, Tetrahedron Lett. 10 (1969) 109-110. |