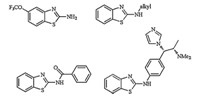
In modern organic synthesis,biologically active benzothiazoles are considered privileged building blocks. In particular 2- aminobenzothiazoles like 2-(N-alkyl amino)benzothiazoles,2- (N-acyl amino)benzothiazoles are involved in several applications in medicinal chemistry [1, 2, 3]. 2-Aminobenzothiazoles are unique scaffolds that are widely used in medicinal and biological chemistry [4]. A large number of 2-anilinobenzothiazole derivatives are found to be anticancer agents and represent an important pharmacophore as well as a reactive intermediate [5]. These compounds have remarkable biological activity and are widely employed for the treatment of various diseases such as tuberculosis [6],epilepsy [7],diabetes [8] and cancer [9] (Fig. 1).
|
Download:
|
| Fig. 1. Biologically active N-substituted-2-aminobenzothiazole derivatives. | |
Due to the pharmaceutical significance of the benzothiazole core structure,several synthetic approaches have been reported. These include coupling of 2-aminobenzothiazoles with aryl halides [10, 11],coupling of aryl amine with 2-halobenzothiazoles [12, 13, 14], arylthioureas with liquid bromine [15],and oxidative cyclization of the intermediates generated by 2-amino thiophenol with isothiocyanates [16]. Recently,direct oxidative coupling between benzothiazoles and amines,Domino three-component reactions of carbon disulfides,amines,2-halo anlines and intermolecular C-S bond formation in the synthesis of 2-aminobenzothiazoles catalyzed by Cu/Pd/Fe have been reported [17, 18, 19, 20, 21, 22]. However, the reported catalytic systems or solvent media are not recyclable. In continuation of our work in cross-coupling reactions and heterogeneous catalysis [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34],herein we describe an inexpensive, air-stable and recyclable nano copper oxide as a catalyst for the synthesis of 2-aminobenzothiazoles under ligand-free conditions (Scheme 1). In the present protocol,PEG-400 (polyethylene glycol) is employed as a low cost and reusable solvent. PEG has received attention of many research groups because it is a hydrophilic polymer. PEG-400 is inexpensive,recyclable,biodegradable, relatively nonflammable andnontoxic,miscible with a wide variety of organic solvents and can be used as an ecofriendly solvent for various organic transformations such as coupling [35], substitution [36],oxidation [37],addition [38] and reduction reactions [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40].
The deployment of PEG in the organic synthesis could reduce the use of volatile organic solvents and environmental burden. PEG can be used as an alternate medium for different reactions with easy recyclability of solvent and catalysts,unlike several of the "neoteric solvents" such as the expensive ionic liquids (ILs). Recently,heterogeneous catalysts have become attractive both from economic and industrial points of view. The high surface area and reactive morphology of the nanomaterials allow them to be effective catalysts for organic synthesis. Nano copper oxides (CuO nps) have the advantages of improved recyclability,easier workup, and cleaner reaction profiles in addition to the lack of necessity of external ligands,which minimizes the organic waste generation,as compared to the conventional catalytic systems. 2. Experimental
All materials were purchased from Sigma Aldrich.Dry solvents and CuO nanopowders (<50 nm) were used for the reactions. Analytical thin layer chromatography (TLC) was carried out using silica gel 60 F254 pre-coated plates. Column chromatography was carried out using silica gel (60-120 mesh size). Visualization was accomplished with UV lamp,I2 stain,and Phosphomolybdic acid charring. 1HNMR and 13C NMR were recorded at 300MHz and 75MHz,respectively,in CDCl3 using TMS as an internal standard. Chemical shifts were reported in parts per million (ppm) downfield from TMS.
General procedure: A mixture of 2-iodoaniline (0.5 mmol), isothiocyanate (0.6 mmol),CuO nanoparticles (0.05 mmol),and Cs2CO3 (1.5 mmol) in PEG-400 (3 mL) was stirred at 80 8C for 8 h (Scheme 1). After the reaction,the reaction mixture was centrifuged and catalyst was separated from the reaction mixture and then followed by washing with ethyl acetate and acetone, dried in vacuo and used directly for further catalytic reactions. The cooled solution was partitioned between ethyl acetate and water, and the organic layer was washed with water and brine,and then dried over Na2SO4. After the removal of the solvent in vacuo,the residue was purified by silica-gel chromatography to give the desired N-substituted-2-aminobenzothiazoles. All the compounds were characterized by comparison with authentic samples [41].

|
Download:
|
| Scheme 1.Recyclable nano CuO catalyzed synthesis of N-substituted-2- aminobenzothiazoles. | |
N-Phenylbenzo[d]thiazol-2-amine (Table 1,entry 1) [40]: White solid,mp 158-160 ℃; yield 180 mg (80%); 1H NMR (300 MHz,CDCl3): δ 8.18-8.04 (m,3H),7.58-7.47 (m,1H),7.46- 7.35 (m,4H),7.37-7.25 (m,1H); 13CNMR(75 MHz,CDCl3): δ 162.4, 157.7,129.3,126.6,124.5,122.0,121.3,120.0,112.6; ESI-MS m/z 227 (M+H)+.
| Table 1 Synthesis of N-substituted-2-aminobenzothiazoles.a |
4,6-Dimethyl-N-phenylbenzo[d]thiazol-2-amine (Table 1,entry 2) [40]: Yield 190 mg (85%); 1H NMR (300 MHz,CDCl3): d 7.52- 7.45 (m,2H),7.41-7.33 (m,2H),7.29-7.25 (m,1H),7.19-7.05 (m, 2H),6.199 (s,1H),2.58 (s,3H) 2.38 (s,3H); 13C NMR (75 MHz, CDCl3): d 201.4,139.9,129.4,128.8,128.2,123.6,119.2,117.9,22.6, 21.2; ESI-MS m/z 255 (M+H)+.
A model reaction was attempted under the optimized reaction conditions with 2-iodoaniline and isothiocyanates as a coupling partner. In general,the reaction proceeded well with these substrates to produce the 2-aminobenzothiazoles in good to excellent yields. It was found that both electron rich and electron poor aryl isothiocyanates underwent the tandem coupling reaction efficiently. The generality of the method was further explored by reacting a wide range of substituted 2-iodoanilines and substituted isothicyanates,resulting in the formation of the corresponding 2- N-substituted benzothiazole derivatives,as shown in (Table 1). All the products were confirmed by 1H NMR,13C NMR spectroscopy, and compared spectroscopically with the authentic samples reported in the literature [41].
Recyclability of the nano copper oxide catalyst was examined under optimized reaction conditions and the results are described in (Table 2). After the reaction,the reaction mixture was centrifuged and catalyst was separated from the reaction mixture and then followed by washing with ethyl acetate and acetone,dried in vacuo and used directly for further catalytic reactions.No significant loss of catalyst activity was observed up to four cycles. The native and used nano copper oxide was analyzed by powder XRD. The powder XRD spectra confirmed that,the peaks of the both fresh and recovered nano copper oxide are identical. It is observed that the morphology of the catalyst remains the same even after four recycls.
| Table 2 Recyclability of CuO nanoparticles.a |
Initially,2-iodo aniline and phenylisothiocyanates were used to optimize the reaction conditions such as base and solvent used and reaction temperature (Table 3),Among several bases screened, Cs2CO3 was found to be an excellent base (Table 3,entry 1). In presence of bases such as K2CO3,Na2CO3,and K3PO4,lesser amount of the desired product was obtained (Table 3,entries 2-4). The effect of solvent was also investigated and the highest yield was observed in PEG-400,while reaction in solvents such as THF, CH3CN,and Toluene resulted in moderate yields (Table 3,entries 6-8). The experiment confirmed that the reaction did not occur in the absence of the base (Table 3,entry 9). When the reaction was conducted at room temperature a lower yield was obtained (Table 3,entry 5). The reaction did not occur in absence of the catalyst (Table 3,entry 10). Ideal temperature for the reaction was found to be 80 8C. The influence of the amount of catalyst on the yield of the product was also evaluated. It was observed that 0.05 mmol of CuO nano was ideal for the synthesis of Nsubstituted- 2-aminobenzothiazoles in good to excellent yields.
| Table 3 Screening of nano copper oxide catalyzed synthesis of N-substituted-2-aminobenzothiazoles.a |
A plausible mechanism,which demonstrates the formation of N-substituted-2-aminobenzothiazoles from the 2-iodoanilines and isothiocyanates,is given in Scheme 2. [D] is formed by the reaction of 2-iodoaniline and isothiocyanate via the de protonation reaction. Then coordination of copper with [D] provides intermediate [E],which upon oxidative additionwould form [F], which undergoes a reductive elimination to produce required final compound [G] with concomitant regeneration of the catalyst.

|
Download:
|
| Scheme 2.Plausible mechanism for the formation of N-substituted-2- aminobenzothiazoles. | |
In conclusion,we have developed a novel protocol for the
synthesis of N-substituted-2-aminobenzothiazoles by nanocrystalline
CuO catalyzed coupling of 2-iodoanilnes and isothiocyanates
under ligand-free conditions in good to excellent yields. This
new protocol underlines the potential use of nanocrystalline CuO
as user friendly,inexpensive,recyclable and efficient catalyst. This
protocol also utilizes efficient,ecofriendly,recyclable,nontoxic,
and biodegradable solvent PEG-400.
Acknowledgment
We thank CSIR,New Delhi,India,for fellowship to GS,KH,KK,
and UGC for fellowship to KR.
| [1] | A.C. Gyorkos, C.P. Corrette, S.Y. Cho, et al., Efficient conversion of substituted aryl thioureas to 2-aminobenzothiazoles using benzyltrimethylammonium tribromide, WO Patent 44 (2005) 793. |
| [2] | A.D. Jordan, C. Luo, A.B. Reitz, A new three-carbon synthon for efficient synthesis of benzannelated and 1-(2-arylethenyl) heterocycles, J. Org. Chem. 68 (2003) 8693-8696. |
| [3] | A.R. Katritzky, D.O. Tymoshenko, D. Monteux, et al., Efficient conversion of substituted aryl thioureas to 2-aminobenzothiazoles using benzyltrimethylammonium tribromide, J. Org. Chem. 65 (2000) 8059-8062. |
| [4] | A.R. Katritzky, D.O. Tymoshenko, D. Monteux, et al., A new three-carbon synthon for efficient synthesis of benzannelated and 1-(2-arylethenyl) heterocycles, J. Org. Chem. 65 (2000) 8059-8062. |
| [5] | I.ĆAleta, M. Kralj, M. Marjanovic, et al., Novel cyano- and amidinobenzothiazole derivatives: synthesis, antitumor evaluation, and X-ray and quantitative structure-activity relationship (QSAR) analysis, J. Med. Chem. 52 (2009) 1744-1756. |
| [6] | H. Suter, H. Zutter, Studien iiber benzthiazole als eventuelle orale antidiabetica, Helv. Chim. Acta 50 (1967) 1084-1086. |
| [7] | V.G. Shirke, A.S. Bobade, R.P. Bhamaria, B.G. Khadse, S.R. Sengupta, Synthesis and antitubercular activity of some new 2-(substituted arylamino)-5,6-disubstituted/ 6-substituted benzothiazoles, Drugs (India) 27 (1990) 350-353. |
| [8] | S.J. Hays, M.J. Rice, D.F. Ortwine, et al., Substituted 2-benzothiazolamines as sodium flux inhibitors: quantitative structure-activity relationships and anticonvulsant activity, J. Pharm. Sci. 83 (1994) 1425-1432. |
| [9] | W. Aelterman, Y. Lang, B. Willemsens, et al., Conversion of the laboratory synthetic route of the N-aryl-2-benzothiazolamine R116010 to a manufacturing method, Org. Process Res. Dev. 5 (2001) 467-471. |
| [10] | Z. Li, S.X. Xiao, G.Q. Tian, et al., Microwave promoted environmentally benign synthesis of 2-aminobenzothiazoles and their urea derivatives, Phosphorus Sulfur Silicon 183 (2008) 1124-1126. |
| [11] | T. Suzuki, S. Igari, A. Hirasawa, Identification of G protein-coupled receptor 120- selective agonists derived from PPARγ agonists, J. Med. Chem. 51 (2008) 7640- 7644. |
| [12] | H.F. Motiwala, R. Kumar, A.K. Chakraborti, Microwave-accelerated solvent- and catalyst-free synthesis of 4-aminoaryl/alkyl-7-chloroquinolines and 2-aminoaryl/ alkylbenzothiazoles, Aust. J. Chem. 60 (2007) 369-374. |
| [13] | F. Delmas, A. Avellaneda, C. Di Giorgio, et al., Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives, Eur. J. Med. Chem. 39 (2004) 685-690. |
| [14] | J. Das, R.V. Moquin, J. Lin, et al., Discovery of 2-amino-heteroaryl-benzothiazole- 6-anilides as potent p56lck inhibitors, Bio. Med. Chem. Lett. 13 (2003) 2587- 2590. |
| [15] | J.M. Sprague, A.H. Land, in: R.C. Elderfield (Ed.), The Chemistry of Heterocyclic Compounds, vol. 5, Wiley, New York, 1957, p. 484. |
| [16] | D. Fajkusova, P. Pazdera, Unexpected formation of benzothiazoles in the synthesis of new heterocycles: benzo-1,2,4-dithiazines, Synthesis 8 (2008) 1297-1305. |
| [17] | L.L. Joyce, G. Evinda, R.A. Batey, Copper- and palladium-catalyzed intramolecular C-S bond formation: a convenient synthesis of 2-aminobenzothiazoles, Chem. Commun. (2004) 446-447. |
| [18] | J.W. Qiu, X.G. Zhang, R.Y. Tang, P. Zhong, J.H. Lia, Iron-catalyzed tandem reactions of 2-halobenzenamines with isothiocyanates leading to 2-aminobenzothiazoles, Adv. Synth. Catal. 351 (2009) 2319-2323. |
| [19] | W. Zhag, Y. Yue, D. Yu, et al., 1,10-Phenanthroline-catalyzed tandem reaction of 2- iodoanilines with isothiocyanates in water, Adv. Synth. Catal. 354 (2012) 2283- 2287. |
| [20] | D. Ma, X. Lu, L. Shi, et al., Domino condensation/S-arylation/heterocyclization reactions: copper-catalyzed three-component synthesis of 2-N-substituted benzothiazoles, Angew. Chem. Int. Ed. 50 (2011) 1118-1128. |
| [21] | R. Xiao, W. Hao, J. Ai, M.Z. Cai, A practical synthesis of 2-aminobenzothiazoles via the tandem reactions of 2-haloanilines with isothiocyanates catalyzed by immobilization of copper in MCM-41, J. Organomet. Chem. 705 (2012) 44-50. |
| [22] | J. Yang, P. Li, L. Wang, Merrifield resin supported phenanthroline Cu(I): a highly efficient and recyclable catalyst for the synthesis of 2-aminobenzothiazoles via the reaction of 2-haloanilines with isothiocyanates, Tetrahedron 67 (2011) 5543- 5549. |
| [23] | K. Swapna, S.N. Murthy, Y.V.D. Nageswar, Copper iodide as a recyclable catalyst for buchwald N-arylation, Eur. J. Org. Chem. (2010) 6678-6682. |
| [24] | K. Swapna, S.N. Murthy, Y.V.D. Nageswar, Recyclable heterogeneous copper oxide on alumina catalyzed coupling of phenols and alcohols with aryl halides under ligand-free conditions, Org. Biomol. Chem. 9 (2011) 5978- 5988. |
| [25] | S.N. Murthy, Y.V.D. Nageswar, O-iodoxybenzoic acid (IBX): a versatile reagent for the synthesis of N-substituted pyrroles mediated by b-cyclodextrin in water, Tetrahedron Lett. 52 (2011) 4481-4484. |
| [26] | K.H.V. Reddy, V.P. Reddy, J. Shankar, et al., Copper oxide nanoparticles catalyzed synthesis of aryl sulfides via cascade reaction of aryl halides with thiourea, Tetrahedron Lett. 52 (2011) 2679-2682. |
| [27] | K. Harsha Vardhan Reddy, G. Satish, V. Prakash Reddy, B.S.P. Anil Kumar, Y.V.D. Nageswar, Recyclable Ru/C catalyzed oxidative cyanation of tertiary amines with TBHP, RSC Adv. 2 (2012) 11084-11088. |
| [28] | K.H.V. Reddy, V.P. Reddy, A.A. Kumar, G. Kranthi, Y.V.D. Nageswar, Nano copper oxide catalyzed synthesis of symmetrical diaryl sulfides under ligand free conditions, Beilstein J. Org. Chem. 7 (2011) 886-891. |
| [29] | K. Swapna, S.N. Murthy, Y.V.D. Nageswar, Nano-CuFe2O4 as a magnetically separable and reusable catalyst for the synthesis of diaryl/aryl alkyl sulfides via cross-coupling process under ligand-free conditions, Org. Biomol. Chem. 9 (2011) 5989-5996. |
| [30] | K. Ramesh, S.N. Murthy, K. Karnakar, et al., A novel bioglycerol-based recyclable carbon catalyst for an efficient one-pot synthesis of highly substituted imidazoles, Tetrahedron Lett. 53 (2012) 1126-1129. |
| [31] | K. Ramesh, S.N. Murthy, Y.V.D. Nageswar, Synthesis of N-substituted pyrroles under catalyst- and solvent-free conditions, Synth. Commun. 42 (2012) 2471- 2477. |
| [32] | K.H.V. Reddy, G. Satish, K. Ramesh, K. Karnakar, Y.V.D. Nageswar, An efficient synthesis of N-substituted indoles from indoline/indoline carboxylic acid via aromatization followed by C-N cross-coupling reaction by using nano copper oxide as a recyclable catalyst, Tetrahedron Lett. 53 (2012) 3061-3065. |
| [33] | K.H.V. Reddy, G. Satish, K. Ramesh, K. Karnakar, Y.V.D. Nageswar, Magnetically separable CuFe2O4 nanoparticle catalyzed C-Se cross coupling in reusable PEG medium, Chem. Lett. 41 (2012) 585-587. |
| [34] | G. Satish, K.H.V. Reddy, K. Ramesh, K. Karnakar, Y.V.D. Nageswar, Synthesis of 2- N-substituted benzothiazoles via domino condensation-hetero cyclization process, mediated by copper oxide nanoparticles under ligand-free conditions,Tetrahedron Lett. 53 (2012), 2521-2521. |
| [35] | D. Srimani, A. Bej, A. Sarkar, Palladium nanoparticle catalyzed Hiyama coupling reaction of benzyl halides, J. Org. Chem. 75 (2010) 4296-4299. |
| [36] | V.V. Namboodiri, R.S. Varma, Microwave-accelerated Suzuki cross-coupling reaction in polyethylene glycol (PEG), Green Chem. 3 (2001) 146- 149. |
| [37] | Z. Hou, N. Thetssen, W. Leitner, Palladium nanoparticles stabilised on PEGmodified silica as catalysts for the aerobic alcohol oxidation in supercritical carbon dioxide, Green Chem. 9 (2007) 127-132. |
| [38] | P.C. Andrews, A.C. Peatt, C.L. Raston, Indium metal mediated synthesis of homoallylic amines in poly(propylene)glycol (PPG), Green Chem. 6 (2004) 119-124. |
| [39] | K.H. Lam, L. Xu, L. Feng, et al., Highly enantioselective iridium-catalyzed hydrogenation of quinoline derivatives using chiral phosphinite H8-BINAPO, Adv. Synth. Catal. 347 (2005) 1755-1759. |
| [40] | W.B. Wang, S.M. Lu, P.Y. Yang, X.W. Han, Y.G. Zhou, Highly enantioselective iridium-catalyzed hydrogenation of heteroaromatic compounds, quinolines, J. Am. Chem. Soc. 125 (2003) 10536-10540. |
| [41] | G. Shen, X. Lv, W. Bao, Synthesis of N-substitut |