Searching for new solid-to-solid phase transitions triggered by temperature is very important not only for the exploration o f novel physical properties but also theoretically for the study of structure-property relationships [1, 2, 3, 4, 5]. Recently,a series of research has been conducted on phase transitions in organic and inorganic hybrid complexes,which possess the properties of both organic and inorganic compounds [6, 7, 8, 9]. Crown ethers are a kind of typical host molecules of organic-inorganic hybrid complexes [10, 11, 12]. In these host-guest compounds,supramolecular packings afford that results in various motions of host and guest components,which can induce a phase transition. An example of phase transition triggered by the motion of guest molecule has been observed in the (m-fluoroanilinium)(dibenzo[18]crown-6)[Ni(dmit)2] crystal,in which the two-fold flip-flop motion ofm-fluoroanilinium guest cation resulted in a ferroelectric-paraelectric phase transition at 346 K by an increase in the temperature [13]. Another notable instance is the supramolecular bola-like ferroelectric 4-methoxyanilinium tetrafluoroborate-18-crown-6,where the origin of the phase transition is due to the order-disorder transition of the pendulum-like motions of the terminal para-methyl group of the 4-methoxyanilinium guest cation [10]. However,examples of phase transition induced by the motion of the host molecule have remained sparse. In our exploration on new phase transition materials [14, 15, 16],we discovered that 2-methoxyanilinium perchlorate-18-crown-6 (1) undergoes a phase transition originating from the order-disorder transition of the host 18-crown-6 molecule. Herein,we study the phase transition property of1by differential scanning calorimetry (DSC),single-crystal X-ray analysis,and dielectric measurements. 2. Experimental
Compound1was readily obtained as colorless block crystals by slow evaporation of the methanol solution containing equal molar 2-methoxyaniline,HClO4-H2O (70%,w/w) and 18-crown-6 at room temperature. The IR spectrum of1shows a strong vibration peak between 1150 cm-1 and 1100 cm-1 ,indicative of the presence of perchlorate anion (Fig. S1 in Supporting information). The powder XRD pattern of1at room temperature matches very well with the pattern simulated from the single-crystal structure (Fig. S2 in Supporting information). The 13C NMR and 1H NMR spectra (Figs. S3 and S4 in Supporting information) were obtained with a Bruker Am-300 spectrometer operating at 300 MHz using DMSO as the solvent at room temperature.
|
Download:
|
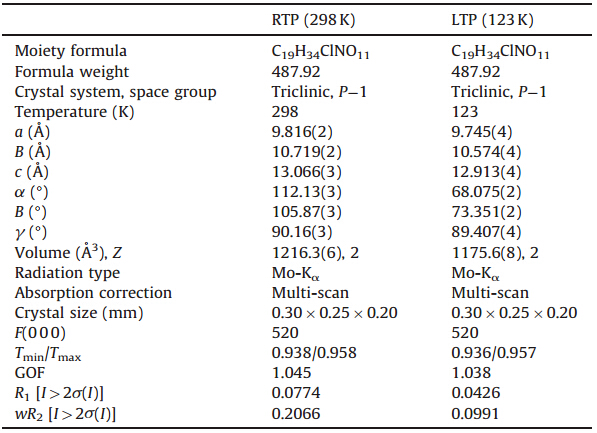
| Fig. 1. DSC curves of1. | |
Generally,DSC measurement is the direct way to detect if this compound displays a reversible phase transition triggered by temperature by seeing the existence of heat anomaly occurring during heating and cooling processes. Upon heating and cooling, the crystalline sample of 1undergoes a single phase transition at approximately Tc= 225 K,showing an endothermic peak at 225 K and an exothermic peak at 210 K (Fig. 1). The sharp peaks reveal the discontinuous character of the transition,indicating a first-order phase transition. From the curve,it is estimated that △H is equal to 1146.612 J/mol while △S is estimated to be △S=△H/Tc= 1146.612/225 = 5.096 J mol-1 K-1. According to Boltzmann’s equationDS=RlnN,where Nis the ratio of possible configurations andRis the gas constant,the Nvalue isca. 1.84, significantly larger than 1,suggesting that the phase transition is an ordered-disordered type and is probably related to order- disorder transition of the host 18-crown-6 molecule.
It is well-known that the phase transition leads not only to the thermal entropy change,but also usually to the dielectric anomaly. Because of the difficulty of growing large crystals,the powder pressed pellet of1was applied to the dielectric measurements. The temperature dependence of the real parte ε'of the dielectric permittivity ε(ε=ε'-jε'') taken at 10,100,and 1000 kHz is shown in Fig. 2. As expected,step-like dielectric anomalies can be observed on these three frequencies during the cooling process around 210 K and during the heating process around 225 K,which matches well with the DSC results.

|
Download:
|
| Fig. 2.Temperature dependencies of the real part of the permittivity of1at 10,100 and 1000 kHz. | |
Variable-temperature single-crystal X-ray diffraction is a common method to reveal microscopic mechanisms of phase transition by investigating the structural changes at the transition. The crystal structures of compound1have been determined at 298 K and 123 K. As shown in Table 1,the room temperature phase (RTP) and low temperature phase (LTP) structures solved in the same triclinic P-1 and the cell parameters show no obvious differences in these two phases. This suggests that the driving force of the phase transition is too weak to induce the change of cell parameters.
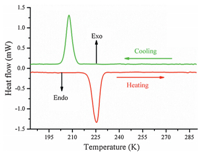
The asymmetric unit of the RTP structure of1contains one 18-crown-6 molecule,one 2-methoxyanilinium cation,and one perchlorate anion (Fig. 3a). The 18-crown-6 molecule is highly orientationally disordered over two positions with occupation factors of 0.581(4) and 0.419(4). To facilitate the explanation,we denote 18-crown-6 with the occupation factor of 0.581(4) by RTPa and 18-crown-6 with the occupation factor of 0.419(4) by RTPb. The mean deviation of the six coplanar oxygen atoms of RTPa and RTPb 18-crown-6 molecules is 0.3591 Å and 0.4530 Å ,respectively. The average values of the relevant bond distances,bond angles,and absolute values of the torsion angles of the RTPa and RTPb 18-crown-6 molecules are similar (Tables S1 and S2 in Supporting information) and compatible with those of 18-crown-6 molecules in other reported host-guest compounds [10, 11, 12].
|
Download:
|
| Fig. 3.The asymmetric unit of1shown at different temperatures. (a) The room temperature phase (298 K): The 18-crown-6 is totally disordered. (b) The low temperature phase (123 K): The 18-crown-6 is totally ordered. Carbon-bound H atoms were omitted for clarity and the intramolecular N-H···O hydrogen bonds are shown as dashed lines (symmetry code: (i)x,-1+y,z).. | |
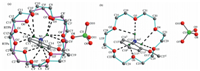
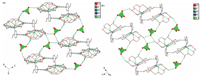
All of the nine non-hydrogen atoms of 2-methoxyanilinium are co-planar with a mean deviation of 0.0167 Å . The ammonium N atom is in the perching position,lying 1.1887 Å and 1.1923 Å from the mean oxygen planes of RTPa and RTPb 18-crown-6 molecules, respectively. The dihedral between the phenyl ring and the mean oxygen plane of the RTPa (RTPb) 18-crown-6 is 88.3418(88.2068), which means the steric repulsion between the 2-methoxy group of 2-methoxyanilinium and 18-crown-6 induced slight deviation from the right dihedral of the phenyl ring with respect to the mean oxygen plane of 18-crown-6. The ammonium moiety was completely included in the cavity of 18-crown-6 by N-H...O hydrogen-bonding interactions (Table S3 in Supporting informa- tion). The hydrogen bonding lengths between the ammonium N atom and the oxygen atom of RTPa and RTPb 18-crown-6 molecules are 2.846(5)-3.119(6) Å and 2.780(9)-3.144(7) Å ,respectively. In addition to intra-molecular hydrogen bonds,C-H···π interactions are the main molecular interaction (Fig. 4a). The C9- H9A···Cg1 interaction can be found between the C9 atom from RTPa 18-crown-6 and the benzene ring,with H9A···Cg1 distance 2.874 Å and C9-H9A···Cg1 angle 126.3388. The C9'-H9' B···Cg1 interaction observed between the C9’atom from RTPb 18-crown-6 and the benzene ring is weaker than the C9-H9 A···Cg1 interac tions with H9’ B···Cg1 distance (3.029 Å ),and the C9’-H9’ B···Cg1 angle (152.5518) is larger. The perchlorate anions,having almost ideal tetrahedral geometry,form weak C-H···Operchloratehydrogen bonds with 18-crown-6 (Table S3). These weak intermolecular C-H···Operchlorate hydrogen bonds and C-H···p interactions together with inter-molecule N-H···O hydrogen bonds links the three parts into a two-dimensional layer (Fig. 5a).

|
Download:
|
| Fig. 4.View of the packings of the organic groups of1at (a) 298 K and (b) 123 K with H···pdistances (Å) and C-H···pangles (8). Dashed lines indicate C-H···pinteractions. | |
In the LTP structure,the content of the asymmetric unit is similar to that in the RTP structure,but the 18-crown-6 (denoted by LTP) is totally ordered (Fig. 3b). The mean deviation of the oxygen plane of LTP 18-crown-6 (0.3573) is similar to that of RTPa but smaller than that of RTPb 18-crown-6. Structural parameters for the LTP 18-crown-6 show little difference from those for the RTPa and RTPb 18-crown-6 molecules (Tables S1 and S2).
|
Download:
|
| Fig. 5.Packing diagrams of1at (a) 298 K and (b) 123 K. Hydrogen bonds and C-H···pinteractions are shown as green and black dashed lines,respectively. | |
| Table 1 Summary of crystallographic data for compound1at 298 K and 123 K |
The mean deviation of the nine coplanar non-hydrogen atoms of 2-methoxyanilinium is 0.0204 Å ,which is a little larger than that in the RTP structure. The dihedral between the phenyl ring and the mean oxygen plane of the 18-crown-6 (84.2178) shows a remarkable decrease,probably because the steric repulsion between the 2-methoxy group of 2-methoxyanilinium and 18-crown-6 increases when the 18-crown-6 is ordered. The intramolecular N-H···O hydrogen bonding interactions are similar to those in the RTP structure,with hydrogen bond length of 2.885(15)- 3.098 (15) Å (see Supporting material Table S3). However, the H···pdistance (2.685 Å ) and C-H···pangle (128.2168)of C24-H24B···Cg2i (symmetry code: (i) x,-1+y,z) interaction shows distinct difference from those of C-H···pinteractions in the RTP structure,indicating the enhancement of C-H···pinteractions (Fig. 4b). The perchlorate anions also form weak C-H···Operchlorate hydrogen bonds with 18-crown-6 (Table S3). The combination of C-H···pinteractions,N-H···O and C-H···Operchlorate hydrogenbonds lead to two-dimensional layers like that in the RTP structure (Fig. 5b).
Hitherto,we have deep insight into the structure change of1.It is clear that the most distinct difference between the RTP and LTP structures is the order-disorder transition of the host 18-crown-6 molecule. Above the phase transition temperature,it can be seen that 18-crown-6 is highly orientationally disordered over two positions. BelowTc,the host 18-crown-6 molecules become totally ordered with the same orientation. In addition,the order-disorder transition of 18-crown-6 changes the C-H···pinteractions and the dihedral between the phenyl ring and the mean oxygen plane of 18-crown-6. Thus,the driving force of the phase transition of1is the order-disorder transition of the host 18-crown-6 molecule, indicating that the phase transition is an ordered-disordered type, which is consistent with the DSC result. Unfortunately,the reorientation of the host 18-crown-6 molecule has not led to symmetry breaking in the low temperature phase,which is different from that found in 4-methoxyanilinium tetrafluoroborate-18-crown-6,where the orientational motions of the guest molecules at low temperature induce the formation of the ferroelectric phase [10]. 4. Conclusion
In summary,we have presented a new host-guest compound,2-methoxyanilinium perchlorate-18-crown-6,which undergoes a reversible phase transition induced by the motion of the host molecule rather than the guest molecule. DSC measurement reveals that the phase transition occurs at 225 K. Dielectric anomalies observed at 225 K (heating) and 210 K (cooling) further confirm the phase transition. The origin of the phase transition was ascribed to the order-disorder transition of the host 18-crown-6 molecule. Supplementary data
CCDC-967207 (298 K) and CCDC-967208 (123 K) contain the supplementary crystallographic data for this paper. This data can be obtained free of charge from the Cambridge Crystallographic Data Centreviawww.ccdc.cam.ac.uk/data_request/cif. Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21101025). Also we gratefully thank Prof. Ren-Gen Xiong for revising this manuscript. Appendix A. Supplementary data
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.025.
| [1] | S. Horiuchi, R. Kumai, Y. Okimoto, Y. Tokura, Chemical approach to neutral-ionic valence instability, quantum phase transition, and relaxor ferroelectricity inorganic charge-transfer complexes, Chem. Phys. 325 (2006) 78-91. |
| [2] | Z. Czapla, S. Dacko, A. Waskowska, Ferroelectric phase transition in hydrogenbonded 2-aminopyridine phosphate (NC4H4NH2)·H3PO4, J. Phys.: Condens. Matter 15 (2003) 3793-3803. |
| [3] | S.G. Li, J.H. Luo, Z.H. Sun, et al., Phase transition triggered by ordering of unique pendulum-like motions in a supramolecular complex: potassium hydrogen bis(- dichloroacetate)-18-crown-6, Cryst. Growth Des. 13 (2013) 2675-2679. |
| [4] | Z.H. Sun, J.H. Luo, S.Q. Zhang, et al., Solid-state reversible quadratic nonlinear optical molecular switch with an exceptionally large contrast, Adv. Mater. 25 (2013) 4159-4163. |
| [5] | Z.H. Sun, X.Q. Wang, J.H. Luo, et al., Ferroelastic phase transition and switchable dielectric behavior associated with ordering of molecular motion in a perovskitelike architectured supramolecular cocrystal, J. Mater. Chem. C 1 (2013) 2561- 2567. |
| [6] | Y. Zhang, W. Zhang, S.H. Li, et al., Ferroelectricity induced by ordering of twisting motion in a molecular rotor, J. Am. Chem. Soc. 132 (2010) 11044-11049. |
| [7] | W. Zhang, H.Y. Ye, H.L. Cai, et al., Discovery of new ferroelectrics:[H2dbco]2·[Cl3] [CuCl3(H2O)2]·H2O (dbco = 1,4-diaza-bicyclo[2.2.2]octane), J. Am. Chem. Soc. 132 (2010) 7300-7302. |
| [8] | Z.H. Sun, J.H. Luo, T.L. Chen, et al., Distinct molecular motions in a switchable chromophore dielectric 4-N,N-dimethylamino-4'-N'-methylstilbazolium trifluoromethanesulfonate, Adv. Funct. Mater. 22 (2012) 4855-4861. |
| [9] | C.M. Ji, Z.H. Sun, S.Q. Zhang, et al., N-Isopropylbenzylammonium tetrafluoroborate: an organic dielectric relaxor with a tunable transition between high and low dielectric states, J. Mater. Chem. C 2 (2014) 567-572. |
| [10] | D.W. Fu, W. Zhang, H.L. Cai, et al., Supramolecular bola-like ferroelectric: 4- methoxyanilinium tetrafluoroborate-18-crown-6, J. Am. Chem. Soc. 32 (2011) 12780-12786. |
| [11] | T. Akutagawa, T. Hasegawa, T. Nakamura, T. Inabe, Supramolecular cation assemblies of hydrogen-bonded (NH4+/NH2NH3+)(crown ether) in [Ni(dmit)2] based molecular conductors and magnets, J. Am. Chem. Soc. 124 (2002) 8903-8911. |
| [12] | T. Akutagawa, D. Sato, Q. Ye, et al., [18]Crown-6 rotator in spin-ladder compound of m-aminoanilinium([18]crown-6)[Ni(dmit)2]-, Dalton Trans. 39 (2010) 8219- 8227. |
| [13] | T. Akutagawa, H. Koshinaka, D. Sato, et al., Ferroelectricity and polarity control in solid-state flip-flop supramolecular rotators, Nat. Mater. 8 (2009) 342-347. |
| [14] | W. Zhang, Y. Cai, R.G. Xiong, H. Yoshikawa, K. Awaga, Exceptional dielectric phase transitions in a perovskite-type cage compound, Angew. Chem. Int. Ed. 49 (2010) 6608-6610. |
| [15] | Y. Zhang, K. Awaga, H. Yoshikawa, R.G. Xiong, Ferroelastic phase transition and dielectric anomalies in 2,4,6-trimethylanilinium perchlorate, J. Mater. Chem. 22 (2012) 9841-9845. |
| [16] | D.W. Fu, H.L. Cai, Y. Liu, et al., Diisopropylammonium bromide is a high-temperature molecular ferroelectric crystal, Science 339 (2013) 425-428. |