Xanthenes exhibit a range of important biological activities such as anti-inflammatory,anti-viral,and anti-bacterial properties [1]. Moreover,due to their spectroscopic properties,xanthenes are used as dyes and fluorescent materials for the visualization of biomolecules [2]. Xanthenones,especially tetrahydroxanthenones, are also an important class of compounds for their distinct structural features and great potential for further transformations [3]. Therefore,the synthesis of xanthene and xanthenone derivatives is of great importance [4]. The development of green and practical methods to construct these skeletons is still highly desired.
Recently,commercially available sodium triphenylphosphinem-sulfonate (TPPMS) was developed as an ion-tagged reagent to mediate a facile Wittig reaction [5]. Afterwards,we demonstrated the use of the TPPMS/CBr4complex as a highly efficient catalyst system for the preparation of acetals from aldehydes,the tetrahydropyranylation of alcohols,and Friedel-Crafts alkylation of indoles with carbonyl compounds to produce bis(indolyl)alkane products (BIAs) (Scheme 1) [6]. These reactions utilize the TPPMS/ CBr4system as a solid complex,which is stable and can be stored easily. The catalyst is soluble in relatively polar organic solvents but can be easily recovered from the reaction mixture by adding a non-polar solvent such as ether. Furthermore,the recovered catalyst can be reused. Multicomponent condensation reaction is a powerful tool for the construction of molecular complexity and structural diversity. Due to their use of one-pot and inherently simple experimental procedure,the development of more sustainable and greener multicomponent reactions is highly desired. As a part of our ongoing program to explore new catalytic activities for this solubility controllable TPPMS/CBr4complex,an efficient one-pot condensation of 2-naphthol with aldehydes to construct 14-aryl(alkyl)-14H-dibenzo[a, j]xanthenes derivatives and one-pot condensation of 2-naphthol with aldehydes and cyclic 1,3-dicarbonyl compounds to construct 12-aryl(alkyl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives is introduced in this paper (Scheme 1).
|
Download:
|
| Scheme 1. Application of easily recoverable TPPMS/CBr4catalyst system. | |
A mixture of 1,3-dicarbonyl compounds (3,1.2 mmol) and TPPMS/CBr4 complex (0.05 mmol) was heated to 100°C; 2-naphthol (1,1 mmol) and the appropriate aldehyde (2,1 mmol) were then added under stirring. After the reaction was completed (as indicated by TLC),DCM (2 mL) and ether (2 mL) were added successively,and the solid TPPMS/CBr4complex was recovered by filtration. The liquid filtrate was evaporated to give the corresponding products. Further purification by recrystallization or flash chromatography was required in some cases. 2.2. Typical procedure for the synthesis of 14-aryl(alkyl)-14Hdibenzo[a,j]xanthenes
A mixture of 2-naphthol (1,2 mmol),the appropriate aldehyde (2,1 mmol),and TPPMS/CBr4complex (0.1 mmol) was stirred at 110°C. After the reaction was completed (as indicated by TLC), DCM (2 mL) and ether (2 mL) were added successively,and the solid TPPMS/CBr4complex was recovered by filtration. The liquid filtrate was evaporated and purified to give the corresponding products. Further purification by recrystallization or flash chromatography was required in some cases. 3. Results and discussion
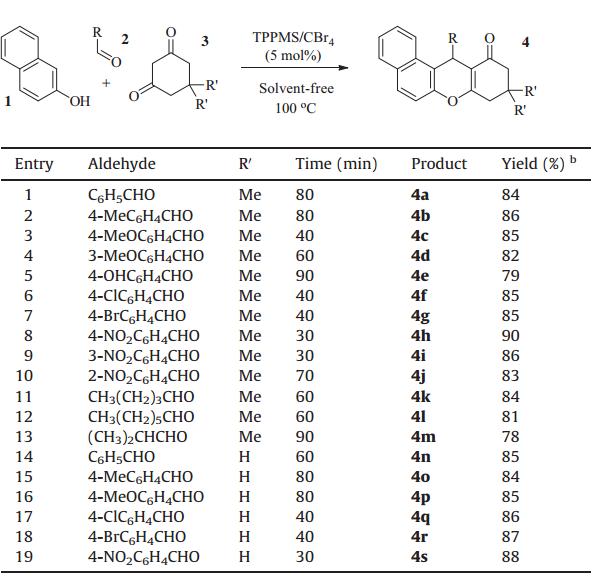
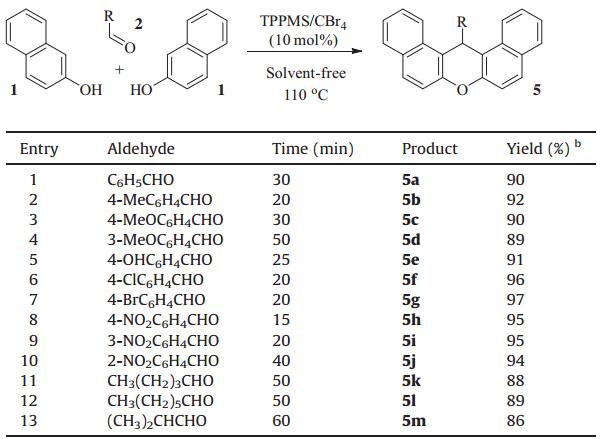
Because a solvent-free protocol leads to a clean,efficient,and economical technology [7],a synthesis using solvent-free conditions was developed. The condensation reaction of 2-naphthol,benzaldehyde,and 5,5-dimethylcyclohexane-1,3-dione was first investigated under solvent-free conditions. After simple optimization of reaction conditions (TPPMS/CBr4 5 mol%,100°C),designed tetrahydrox-anthnone derivative 4a was delivered with high yield (Table 1,entry 1). With the optimized conditions in hand,we then explored the generality and scope of this TPPMS/CBr4catalyzed transformation. The reactivity of various aldehydes was first examined as summarized in Table 1. In general,the TPPMS/CBr4complex was able to catalyze the reaction of both aryl and aliphatic aldehydes. Aryl aldehydes with electron-donating substituents and with electronwithdrawing substituents were all tolerant of the reaction conditions (Table 1,entries 2-10). The aldehydes withorthoormetasubstituents delivered the corresponding xanthenones in high yields as well (Table 1,entries 4,9,10). The unprotected phenol group was tolerated in this reaction (Table 1,entry 5). The introduction of a halogen atom into this system made the methodology more useful for further transformation (Table 1,entries 6,7). Aliphatic aldehydes also gave good yields of the corresponding xanthenones (Table 1,entries 11- 13). The use of 1,3-cyclohexanedione in place of 5,5-dimethyl-1,3-cyclohexanedione also gave similar results,as shown in Table 1, entries 14-19. Encouraged by these good results,we next tested the condensation reaction of 2-naphthol with benzaldehyde to construct xanthene derivatives. After slight tuning of the reaction conditions (TPPMS/CBr410 mol%,110°C),the desired product 5a was achieved with complete conversion and an excellent isolated yield (Table 2, entry 1). The generality and scope of this transformation was also investigated,as shown in Table 2.
| Table 1 Synthesis of 12-aryl(alkyl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-onesa. |
| Table 2 Synthesis of 14-aryl(alkyl)-14H-dibenzo[a,j]xanthenesa. |
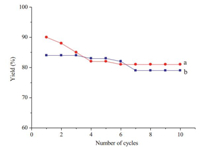
In both of the reactions,the separation and recovery of the catalyst system was simply carried out by precipitation with DCM and ether after the reaction. The recovered catalyst could be reused without loss of catalytic activity. We demonstrated this with the reaction of 2-naphthol with benzaldehyde (Fig. 1,curve a) and the reaction of 2-naphthol with benzaldehyde and 5,5-dimethylcyclohexane-1,3-dione (Fig. 1,curve b) using recovered TPPMS/CBr4 system for ten cycles without distinctly diminished yield. To evaluate the practicality of our method [8],the reaction between 2-naphthol and benzaldehyde and the reaction between 2-naphthol,benzaldehyde and 5,5-dimethylcyclohexane-1,3-dione were performed on a larger scale (50 mmol) in a single batch,and no yield loss was observed (83%,88% isolated yield respectively). That is to say,herein we present a practical and scalable synthetic entry to the xanthene and xanthenone derivatives.
|
Download:
|
| Fig. 1. Studying reusablility of TPPMS/CBr4 in the synthesis of xanthenes and xanthenones. | |
In conclusion,we have demonstrated that the TPPMS/CBr4 complex is a highly efficient catalyst for the preparation of 14-aryl(alkyl)-14H-dibenzo[a, j]xanthenes and 12-aryl(alkyl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones under solventfree conditions. The catalyst is stable and can be stored easily.It is easily separable and can be recovered and reused without loss of catalytic activity for 10 cycles. Further applications of TPPMS/ CBr4complex on the extension of this protocol are ongoing in our group. Acknowledgments
We thank the National Natural Science Foundation of China (Nos. 21002080,21262029) and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20106203120003) of the Education Ministry for financially supporting this work.
| [1] | (a) A. Kumar, S. Sharma, R.A. Maurya, J. Sarkar, Diversity oriented synthesis of benzoxanthene and benzochromene libraries via one-pot, three-component reactions and their antiproliferative activity, J. Comb. Chem. 12 (2010) 20-24; (b) M.V. Encinas, A.M. Rufs, S.G. Bertolotti, C.M. Previtali, Xanthene dyes/amine as photoinitiators of radical polymerization: a comparative and photochemical study in aqueous medium, Polymer 50 (2009) 2762-2767; (c) Y.Z. Zhang, H. Gorner, Photoprocesses of xanthene dyes bound to lysozyme or serum albumin, Photochem. Photobiol. 85 (2009) 677-685; (d) F.M. Muñiz, V. Alcázar, L. Simón, et al., Daxabe—A xanthene-based fluorescent sensor for 3,5-dinitrobenzoic acid and anions, Eur. J. Org. Chem. (2009) 1009-1015; (e) M. Sibrian-Vazquez, R. Strongin, Optimising the synthesis and red-green-blue emission of a simple organic dye, Supramol. Chem. 21 (2009) 107-110. |
| [2] | (a) N. Saki, T. Dinc, E.U. Akkaya, Excimer emission and energy transfer in cofacial boradiazaindacene (BODIPY) dimers built on a xanthene scaffold, Tetrahedron 62 (2006) 2721-2725; (b) C.G. Knight, T.E. Stephens, Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles, Biochem. J. 258 (1989) 683-687. |
| [3] | (a) B. Lesch, S. Bräse, A short, atom-economical entry to tetrahydroxanthenones, Angew. Chem. Int. Ed. 43 (2004) 115-118; (b) Y.L. Shi, M. Shi, Reaction of salicyl N-tosylimines with 2-cyclohexenone: a facile access to tetrahydroxanthenones, Synlett (2005) 2623-2626. |
| [4] | (a) J.J. Li, W.Y. Tang, L.M. Lu, W.K. Su, Strontium triflate catalyzed one-pot condensation of b-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds, Tetrahedron Lett. 49 (2008) 7117-7120; (b) B. Das, K. Laxminarayana, M. Krishnaiah, Y. Srinivas, An efficient and convenient protocol for the synthesis of novel 12-aryl- or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives, Synlett (2007) 3107-3112; (c) E. Böβ, T. Hillringhaus, J. Nitsch, M. Klussmann, Lewis acid-catalysed one pot synthesis of substituted xanthenes, Org. Biomol. Chem. 9 (2011) 1744-1748; (d) P.V. Shinde, A.H. Kategaonkar, B.B. Shingate, M.S. Shingare, Surfactant catalyzed convenient and greener synthesis of tetrahydrobenzo[a]xanthene-11-ones at ambient temperature, Beilstein, J. Org. Chem. 7 (2011) 53-58; (e) R. Kumar, G.C. Nandi, R.K. Verma, M.S. Singh, A facile approach for the synthesis of 14-aryl- or alkyl-14H-dibenzo[a,j]xanthenes under solvent-free condition, Tetrahedron Lett. 51 (2010) 442-445. |
| [5] | (a) C.D. Huo, X. He, T.H. Chan, Zwitterionic phosphonium sulfonates as easily phase-separable ion-tagged Wittig reagents, J. Org. Chem. 73 (2008) 8583-8586; (b) C. Huo, T.H. Chan, A novel liquid-phase strategy for organic synthesis using organic ions as soluble supports, Chem. Soc. Rev. 39 (2010) 2977-3006. |
| [6] | (a) C. Huo, T.H. Chan, Carbon tetrabromide/sodium triphenylphosphine-m-sulfonate (TPPMS) as an efficient and easily recoverable catalyst for acetalization and tetrahydropyranylation reactions, Adv. Synth. Catal. 351 (2009) 1933-1938; (b) C.D. Huo, C. Sun, C. Wang, X. Jia, W. Chang, Triphenylphosphine-m-sulfonate/ carbon tetrabromide as an efficient and easily recoverable catalyst system for Friedel-Crafts alkylation of indoles with carbonyl compounds or acetals, ACS Sust. Chem. Eng. 1 (2013) 549-553; (c) C.D. Huo, C. Wang, D.C. Hu, X.A. Jia, Clean and efficient conversion of aldehydes into the corresponding nitriles using ionic supported triphenylphosphine and CBr4, Lett. Org. Chem. 10 (2013) 442-444. |
| [7] | (a) J. Azizian, F. Thimouri, F.M. Mohammadizadeh, Ammonium chloride catalyzed one-pot synthesis of diindolylmethanes under solvent-free conditions, Catal. Commun. 8 (2007) 1117-1121; (b) K. Tanaka, F. Toda, Solvent-free organic synthesis, Chem. Rev. 100 (2000) 1025- 1074; (c) P. Lidstrom, J. Tierney, B. Wathey, J. Westman, Microwave assisted organic synthesis - a review, Tetrahedron 57 (2001) 9225-9283; (d) A. Loupy, A. Petit, J. Hamelin, et al., New solvent-free organic synthesis using focused microwaves, Synthesis (1998) 1213-1234. |
| [8] | (a) A. Mendoza, Y. Ishihara, P.S. Baran, Scalable enantioselective total synthesis of taxanes, Nat. Chem. 4 (2012) 21-25; (b) C.D. Huo, X.L. Xu, J.Z. An, et al., Approach to construct polysubstituted 1,2- dihydronaphtho[2,1-b]furans and their aerobic oxidative aromatization, J. Org. Chem. 77 (2012) 8310-8316. |