In recent years,fluorescent polymers [1, 2, 3, 4] have attracted great attention because of their intrinsic advantages,including low toxicity,good biocompatibility,long-term stability and facile conjugation with functional molecules. Fluorescent polyurethanes are some of the most popular polymers because of their enormous diversity of chemical compositions and properties,and their importance not only in textiles,coating materials and paper making,but also in organic LEDs and fluorescent probes [5, 6, 7].
Related copolymers have been reported; for example,Wanget al. [8] synthesized some new types of fluorescent dye-based polyurethane ionomers. Kim et al. [9] developed fluorescent polyurethane foams containing a red fluorescent perylene chromophore. However,to date,there have been very few reports investigating the synthesis and properties of fluorescent siloxanepolyurethanes. Siloxane-polyurethanes are high-performance materials that combine the excellent mechanical properties of conventional polyurethanes with the high elasticity,and good thermal and biological stability of polysiloxanes [10, 11, 12]. In particular,siloxane-polyurethanes possess excellent bio- and blood compatibility,so that they can be used for medical devices, such as artificial hearts,intra-aortic balloons,pacemaker leads, heart valves and hemodialysis membranes [13, 14].
1,8-Naphthalimide monomers are interesting compounds because their useful photophysical and biological properties show promise for medical applications as free radical scavengers, potential photoredox anticancer agents,fluorescent labels, photosensitizers,and imaging agents [15, 16, 17]. In addition,they are particularly attractive fluorophores because they can exhibit high quantum yields,good photostability and their fluorescence can be tuned throughout a wide spectral range from blue to red [18]. The attractive properties of 1,8-naphthalimide monomers have led to their incorporation into numerous polymers to tailor solubility,self-association,and molecular size to suit specific applications [19, 20]. However,the direct use of 1,8-naphthalimide monomers is unfavorable because they are difficult to immobilize,easy to wash off and not resistant to solvents. As a result,chemical bonding of 1,8-naphthalimide monomers into polymermatrixestoaffordfluorescentpolymershasbeen attempted [21, 22, 23]. In general,because 1,8-naphthalimide monomers contain few hydrophilic groups,they do not react readily with 4,4'-methylenediphenyl diisocyanate (MDI) or other diisocyanates and do not disperse well in normal resins,and therefore,novel fluorescent monomers with more reactive groups need to be developed.
In this paper,4-bromo-1,8-naphthalic anhydride was chosen as a starting material to react with ethylenediamine to form a novel amino-functionalized,fluorescent monomer,2-(2-aminoethyl)-6-((2-aminoethyl)amino)-1H-benzo[de]isoquinoline-1, 3(2H)-dione (AABD). Fluorescent siloxane-polyurethanes (HPMSPUs) were synthesized by attaching different amounts of AABD as a chain extenderviaa two-step process. The content of AABD segments in the synthesized HPMS-PUs was investigated by ultraviolet-visible (UV-vis) spectroscopy and the fluorescent properties of HPMS-PUs were characterized by fluorometry. The thermal stability and thermal migration properties of the HPMSPUs were also studied. 2. Experimental 2.1. Materials
MDI,4-bromo-1,8-naphthalic anhydride and rhodamine 6G were purchased from Aldrich Chemical Company (Shanghai, China),and MDI was vacuum distilled before use. Hydroxylterminated polydimethylsiloxane (HPMS,Mn= 1800,hydroxyl number = 62.3 mg KOH/g) was synthesized by hydrosilylation of hydrogen-terminated polydimethylsiloxane (Mn= 1000) with allyl polyethylene oxide (Mn= 400) using Wilkinson and Karstedt catalysts [24]. Hydrogen-terminated polydimethylsiloxane and allyl polyethylene oxide were supplied by Zhongshan Chemical Co.,Ltd. (Jiangsu,China). Trimethylolpropane (TMP),N-methyl-2-pyrrolidone (NMP),ethylenediamine,acetone and hydroquinone were all reagent grade and purchased from Sinopharm Chemical Reagent Co.,Ltd. (Beijing,China). 2.2. Synthesis of AABDM
The synthesis of AABD is shown in Scheme 1. 4-Bromo-1,8-naphthalic anhydride (2.50 g,9.02 mmol) was dissolved in a mixture of ethanol (40 mL) and ethylenediamine (60 mL) and the resulting solution was heated under reflux for 10 h. The solution was allowed to cool and then the ethylenediamine was distilled under vacuum to afford a crude product. The pure compound AABD was obtained after silica gel chromatography using dichloromethane as the eluent. Yield 92%,mp 234.4-235.7°C; 1H NMR (400 MHz,DMSO-d6): δ (ppm) = 8.52-8.55 (d,1H, J= 8.72 Hz,Ar-H),8.32-8.34 (d,1H,J= 7.16 Hz,Ar-H),8.09-8.11 (d,1H,J= 8.28 Hz,Ar-H),7.60-7.64 (t,1H,J= 7.48 Hz,Ar-H),6.73- 6.75 (d,1H,J= 8.44 Hz,Ar-H),4.83 (s,4H,-NH2),4.41 (s,1H,-NH- ),4.04-4.07 (t,J=6.20Hz,2H,-CH2-),3.93-3.96 (t,2H, J=5.24Hz,-CH2-),3.22-3.25 (t,2H,J=5.24Hz,-CH2-),2.99- 3.02 (t,2H,J=6.20Hz,-CH2-). Anal. Calcd. for C16H18N4O2(%): C, 64.41; H,6.08; N,18.78. Found: C,64.49; H,6.04; N,18.71. TOFMS:m/z299.34 [M+1]+.

|
Download:
|
| Scheme 1.Synthesis of AABD. | |
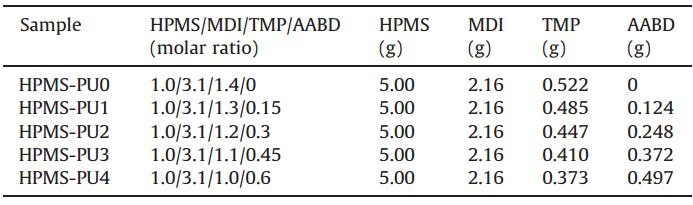
A series of HPMS-PUs were prepared by a two-step bulk polymerization without catalyst,as depicted in Scheme 2. All glassware was dried in an oven overnight at 100°C prior to use. Prepolymer preparation:HPMS (5.00 g,2.78 mmol) was charged into a 100-mL four-necked flask equipped with a mechanical stirrer,nitrogen inlet,reflux condenser and thermometer. MDI (2.16 g,8.63 mmol) was dissolved in acetone (15 mL) and added dropwise over a period of 30-60 min maintaining the temperature below 40°C. Then,the mixture was reacted at 80°C for 2 h under N2to obtain an isocyanate-terminated polymer with an isocyanate content of 6.5 wt%,which was quantitatively determined by the din-butylamine method [25]. Chain extension: Following the above-mentioned process,a suitable amount of acetone was added to the system to reduce its viscosity. First,AABD with a stoichiometric ratio as listed in Table 1,was added to the reactor at 80°C and stirred vigorously for 2 min. The appropriate amount of TMP was added and the mixture stirred vigorously for 2 min. Then,the reaction mixture was distilledin vacuo to ensure the removal of air bubbles and excess acetone. Finally,the mixture was cast in a preheated Teflon mold to form a film with a thickness of 100-200mm,which was cured for 24 h at 60°C.

|
Download:
|
| Scheme 2.Preparation of HPMS-PUs. | |
| Table 1 Compositions of HPMS-PUs prepared in this study. |
The 1H NMR spectra of AABD were measured in DMSO-d6 using TMS as an internal standard on a Bruker AV-400 spectrometer with chemical shifts reported in ppm. UV-vis spectra of AABD and HPMS-PUs were recorded on a Varian Cary double beam spectrometer using a quartz cell with a pathlength of 10 mm. The scanned wavelength ranged from 350 nm to 550 nm, with a medium scanning rate used. Fluorescence spectra of AABD and HPMS-PUs were recorded on a SPEX Fluoromax-3 spectrofluorometer. All emission spectra were measured in quartz cuvettes with a pathlength of 10 mm using lex= 415 nm. Emission data were collected from 425 nm to 700 nm (increments of 1 nm) using 1-nm slit widths in excitation and emission (wavelength resolution of 1 nm),and corrected for nonlinear instrument response. The thermal stability of HPMS-PUs was investigated by thermogravimetric analysis (TGA) using a TGA 2050 (TA Instruments). The heating rate was 10°C/min and each sample was heated to 600°C in air. The weight of each sample was 5-10 mg. 3. Results and discussion 3.1. UV-vis spectra
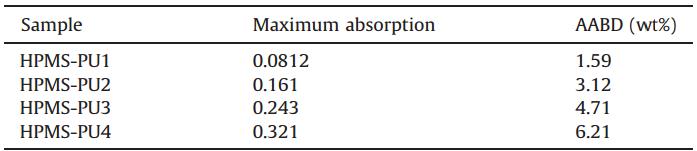
UV-vis spectra were used to measure the content of AABD segments in the synthesized HPMS-PUs. To obtain a standard curve,first,different amounts of AABD were dissolved in NMP. Then the UV-vis absorption spectra of these AABD solutions with different concentrations were recorded as presented in Fig. 1a. We recorded the response of the maximum absorption and AABD concentration,as shown in the inset of Fig. 1a. As a result, the following curve fitting equation could be obtained:

Then,20 mg of each HPMS-PU was dissolved in NMP,and each solution was made up to 100 mL in a volumetric flask. The UV-vis absorption spectra of the HPMS-PUs were then measured,as illustrated in Fig. 1b. Here,the weight percentages of AABD segments in HPMS-PU1-4 were determined by Eq. (1) and results listed in Table 2. Compared with the UV-vis absorption spectrum of HPMS-PU0 without AABD,those of the HPMS-PUs showed an absorption peak at 430 nm caused by covalent bonding with AABD. The maximum absorption wavelengths of AABD and HPMS-PUs were 426 and 430 nm,respectively,so the HPMS-PUs showed a red shift of about 4 nm. Compared with the fluorescent monomer,the increased number of conjugated groups in the polyurethane chains increased the degree of electron delocalization and decreased the energy of thep-p* transition,so the absorption shifted to longer wavelength [26].

|
Download:
|
| Fig. 1. (a) UV-vis absorption spectra of different concentrations of AABD in NMP. Inset: a linear relationship is obtained between the maximum absorption and AABD concentration (10-5 mol/L). (b) UV-vis absorption spectra of HPMS-PUs in NMP. | |
| Table 2 Content of AABD segments in synthesized HPMS-PUs. |
To evaluate the fluorescent properties of HPMS-PUs,their fluorescence spectra were measured. The shape of fluorescence from HPMS-PUs was similar and the maximum emission wavelength was the same,so the fluorescence emission spectrum of HPMS-PU2 was chosen as a representative example to study the fluorescent properties of the HPMS-PUs. As shown in Fig. 2a,the intensity of fluorescence of HPMS-PU2 in NMP was higher than that of AABD with the same fluorophore concentration. Compared with AABD in NMP,the maximum emission wavelength of HPMSPU2 showed a blue shift of about 9 nm. Fig. 2b depicts images of HPMS-PU0 and HPMS-PU2 films taken under both daylight and a 365-nm ultraviolet lamp. HPMS-PU2 displayed intense green fluorescence compared with HPMS-PU0. HPMS-PU0 without AABD showed no light emission within the range of 425-700 nm.

|
Download:
|
| Fig. 2. (a) Fluorescence emission spectra of AABD,HPMS-PU0 and HPMS-PU2 in NMP. AABD and HPMS-PU2 in NMP had the same fluorophore concentration of 2×10-4mol/ L. (b) Optical images of HPMS-PU0 and HPMS-PU2 taken under daylight and a 365-nm UV lamp. | |
It is well known that Stokes shift (Δν) and fluorescence quantum yield are important parameters of fluorescent monomers. TheΔν can indicate the difference in structure and properties of fluorescent monomers between the ground stateS0 and first excited stateS1. The shift Δνcan be calculated according to the following equation [19]:

Fluorescence quantum yield is used to evaluate the ability of fluorescent monomers to emit absorbed light energy. The fluorescence quantum yield of AABD and HPMS-PU2 in NMP was determined using rhodamine 6G as a standard. The quantum yields of AABD and HPMS-PU2 were calculated using the equation [28]:

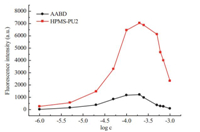
To determine if concentration self-quenching [26] occurred in the HPMS-PUs,the fluorescence emission spectra of AABD and HPMS-PU2 over a broad concentration range of 1×10-6to 1×10-3mol/L were recorded. As shown in Fig. 3,the fluorescence intensity of both AABD and HPMS-PU2 initially increased as the concentration increased,and then decreased when the concentration exceeded 2×10-4mol/L. This confirms that concentration self-quenching occurred in both AABD and HPMS-PU2 through aggregation of the fluorophores [29]. Furthermore,the fluorescence intensity of HPMS-PU2 decreased less than that of AABD with increasing concentration. The reason for this is that the high molecular weight of HPMS-PUs could slow down the motion of the molecules and limit the aggregation of the fluorophores.
|
Download:
|
| Fig. 3. Fluorescence intensity of AABD and HPMS-PU2 in NMP in a broad concentration range. | |
The stability of fluorescence of HPMS-PUs was also investigated. The fluorescence spectra of AABD and HPMS-PU2 with the same fluorophore concentration measured at different temperatures are shown in Fig. 4. The intensity of fluorescence of AABD and HPMSPU2 decreased with rising temperature because of the effect of energy transformation. On the one hand,the interaction between the fluorophores and solvent molecules increased as the temperature increased,which resulted in energy loss [21]. On the other hand,the high molecular weight of HPMS-PUs could slow the motion of the molecules and decrease molecular collision. Overall, these two factors caused the fluorescence intensity of HPMS-PU2 to decrease with increasing temperature,although the fluorescence intensity of HPMS-PU2 decreased much less than that of AABD. This indicates that the fluorescence stability of HPMS-PUs is greater than that of AABD. Moreover,the fluorescence of HPMSPUs showed quite good stability during storage. After one month at ambient temperature,the fluorescence spectra of HPMS-PUs were similar to those observed from the fresh solutions.

|
Download:
|
| Fig. 4. Fluorescence emission spectra of (a) AABD,and (b) HPMS-PU2 at different temperatures with the same fluorophore concentration of 1×10-4mol/L. | |
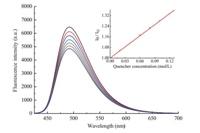
The fluorescence stability of HPMS-PU2 was also confirmed using a fluorescence quencher. According to a published method [30, 31, 32],hydroquinone was used as a general quencher to analyze fluorescence stability. Fig. 5 shows the fluorescence emission spectra of HPMS-PU2 in NMP with hydroquinone as a quencher.The fluorescence quenching efficiency could be calculated according to the Stern-Volmer equation:

|
Download:
|
| Fig. 5. Fluorescence emission spectra of HPMS-PU2 quenched by hydroquinone. Inset: plot of I0/IQ versusthe concentration of hydroquinone [Q]. | |
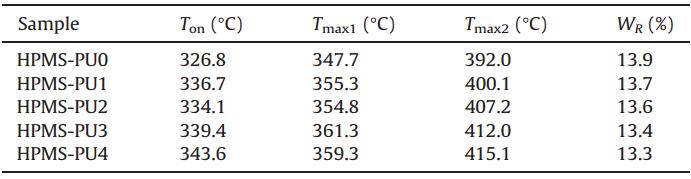
TGA and differential thermogravimetric (DTG) curves for HPMSPUs with different contents of AABD are presented in Fig. 6. Detailed data of Ton(the temperature at 5% weight loss),Tmax1 (maximumrate of degradation temperature in the first stage),Tmax2 (maximum rate of degradation temperature in the second stage) and WR (residue at 600°C) are summarized in Table 3. Generally,the first decomposition stage of polyurethane chains is of the hard segments,which includes the urea and urethane groups [33]. The second decomposition stage involves loss of the soft segments,such as polyester and polyether. With regards to the other components,polysiloxanes are known to exhibit high thermal stability up to 300°C [34]. This stability is a result of the presence of Si-O bonds,which have a higher dissociation energy (460.5 kJ/mol) than C-O (358.0 kJ/mol) and C-C (304.0 kJ/mol) bonds [35, 36]. Table 3 data reveals that the Tonof all HPMS-PUs is up to 325°C because of the good thermal stability of the HPMS component. Ton,Tmax1 and Tmax2of the HPMS-PUs increased with the content of AABD,and the incorporation of AABD increased Tmax2by as much as 23°C,which might be ascribed to the rigid naphthalene ring structure of the chain extender AABD. In addition, the amount of solid residue after degradation of HPMS-PUs was consistent with the content of siloxane in HPMS.

|
Download:
|
| Fig. 6. (a) TGA curves and (b) DTG curves of HPMS-PUs. | |
| Table 3 TGA and DTG results for HPMS-PUs. |
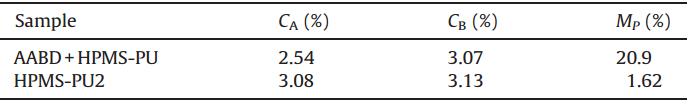
The American Association of Textile Chemists and Colorists (AATCC) method [37] was used to test the thermal migration properties of the fluorescent monomers using HPMS-PU2 as a representative example. First,a composite film of AABD and HPMSPU was prepared using a blend of HPMS-PU0 with AABD,where the AABD content was 3.12 wt%,which is the same as the AABD content of HPMS-PU2. The polyurethane film was covered with a watch glass with a diameter of 60 mm (region A). After keeping the temperature at 60°C for 24 h,the same weight of each of region A and uncovered film (region B) were dissolved in NMP,and then the UV-vis absorption of the solutions was measured. The migration property (MP) could be calculated using the equation:

| Table 4 Thermal migration properties of polyurethane films. |
In summary,a series of HPMS-PUs containing different amounts of AABD as a chain extender were synthesized. The chemical structure of AABD was confirmed by 1H NMR spectroscopy. The content of AABD in the HPMS-PUs was investigated by UV-vis spectroscopy and the fluorescent properties of HPMS-PUs were characterized by fluorometry. Compared with AABD in NMP, the maximum absorption wavelength of HPMS-PUs showed a red shift of about 4 nm,while the maximum emission wavelength of HPMS-PUs showed a blue shift of about 9 nm. The Stokes shifts,Δν, of AABD and HPMS-PU2 were 3514 and 2931 cm-1,respectively. The quantum yield of HPMS-PU2 was 0.79,which was six times higher than that of AABD. Concentration self-quenching occurred in both AABD and HPMS-PUs through the aggregation of the fluorophores. The fluorescence of HPMS-PUs showed good stability with respect to both temperature and the effects of a fluorescence quencher. The thermal stability of HPMS-PUs increased with the content of AABD,and the incorporation of AABD increased Tmax2by as much as 23°C. The fluorophore units of the HPMS-PUs did not migrate readily; the fluorophoreMPof HPMS-PU2 was only 1.62%. Acknowledgment
Financial support from the 863 program (No. 2011AA02A204) is acknowledged.
| [1] | G. Moad, M. Chen, M. Häussler, et al., Functional polymers for optoelectronic applications by RAFT polymerization, Polym. Chem. 2 (2011) 492-519. |
| [2] | T. Klingstedt, K.P.R. Nilsson, Conjugated polymers for enhanced bioimaging, Biochim. Biophys. Acta - Gen. Subjects 1810 (2011) 286-296. |
| [3] | D. Liu, Y.H. Duan, Synthesis of novel thieno-[3,4-b]-pyrazine-cored molecules as red fluorescent materials, Chin. Chem. Lett. 24 (2013) 809-812. |
| [4] | J.H. Kim, K. Park, H.Y. Nam, et al., Polymers for bioimaging, Prog. Polym. Sci. 32 (2007) 1031-1053. |
| [5] | M.O. Liu, H.F. Lin, M.C. Yang, et al., Thermal and fluorescent properties of optical brighteners and their whitening effect for pelletization of cycloolefin copolymers, Mater. Lett. 60 (2006) 2132-2137. |
| [6] | L. Torini, J.F. Argillier, N. Zydowicz, Interfacial polycondensation encapsulation in miniemulsion, Macromolecules 38 (2005) 3225-3236. |
| [7] | K. Kojio, Y. Mitsui, M. Furukawa, Synthesis and properties of highly hydrophilic polyurethane based on diisocyanate with ether group, Polymer 50 (2009) 3693- 3697. |
| [8] | C.L. Wang, Z.J. Zhang, Y.M. Kuo, D.Y. Chao, Fluorescence from fluorescent dyebased polyurethane ionomer (Ⅱ), Polym. Adv. Technol. 15 (2004) 93-99. |
| [9] | O. Kim, M.S. Gong, Fluorescent polyurethane foams containing red fluorescent perylene chromophore, J. Ind. Eng. Chem. 10 (2004) 801-805. |
| [10] | M.M. Rahman, A. Hasneen, H.D. Kim, W.K. Lee, Preparation and properties of polydimethylsiloxane (PDMS)/polytetramethyleneadipate glycol (PTAd)-based waterborne polyurethane adhesives: effect of PDMS molecular weight and content, J. Appl. Polym. Sci. 125 (2012) 88-96. |
| [11] | J.P. Sheth, A. Aneja, G.L. Wilkes, et al., Influence of system variables on the morphological and dynamic mechanical behavior of polydimethylsiloxane based segmented polyurethane and polyurea copolymers: a comparative perspective, Polymer 45 (2004) 6919-6932. |
| [12] | J.C. McDonald, G.M. Whitesides, Poly(dimethylsiloxane) as a material for fabricating microfluidic devices, Acc. Chem. Res. 35 (2002) 491-499. |
| [13] | G. Kwak, T. Masuda, Poly(silyleneethynylenephenylene) and poly(silylenephenyleneethynylenephenylene) s: Synthesis and photophysical properties related to charge transfer, Macromolecules 35 (2002) 4138-4142. |
| [14] | G. Kwak, A. Takagi, M. Fujiki, T. Masuda, Facile preparation of transparent, homogeneous, fluorescent gel film based on s-p-conjugated, hyperbranched polymer with siloxane linkages by means of hydrosilylation and aerial oxidation, Chem. Mater. 16 (2004) 781-785. |
| [15] | O. Jaudouin, J.J. Robin, J.M. Lopez-Cuesta, D. Perrin, C. Imbert, Ionomer-based polyurethanes: a comparative study of properties and applications, Polym. Int. 61 (2012) 495-510. |
| [16] | Y. Zhang, S.B. Feng, Q. Wu, et al., Microwave-assisted synthesis and evaluation of naphthalimides derivatives as free radical scavengers, Med. Chem. Res. 20 (2011) 752-759. |
| [17] | J.E. Rogers, L.A. Kelly, Nucleic acid oxidation mediated by naphthalene and benzophenone imide and diimide derivatives: consequences for DNA redox chemistry, J. Am. Chem. Soc. 121 (1999) 3854-3861. |
| [18] | H.B. Xiao, M.J. Chen, G.H. Shi, et al., A novel fluorescent molecule based on 1,8- naphthalimide: synthesis, spectral properties, and application in cell imaging, Res. Chem. Intermed. 36 (2010) 1021-1026. |
| [19] | I. Grabchev, S. Sali, R. Betcheva, V. Gregoriou, New green fluorescent polymer sensors for metal cations and protons, Eur. Polym. J. 43 (2007) 4297-4305. |
| [20] | I. Grabchev, V. Bojinov, Synthesis and characterisation of fluorescent polyacrylonitrile copolymers with 1,8-naphthalimide side chains, Polym. Degrad. Stabil. 70 (l) (2000) 47-153. |
| [21] | Y. Wang, X.G. Zhang, B. Han, et al., The synthesis and photoluminescence characteristics of novel blue light-emitting naphthalimide derivatives, Dyes Pigments 86 (2010) 190-196. |
| [22] | Y.Y. Zhang, C.H. Zhou, Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents, Bioorg. Med. Chem. Lett. 21 (2011) 4349-4352. |
| [23] | Y.C. Chen, R.R. Chiou, H.L. Huang, et al., Fluorescence from fluorescent dye based polyurethane ionomer (Ⅲ), J. Appl. Polym. Sci. 97 (2005) 455-465. |
| [24] | J.V. Crivello, B. Daoshen, Regioselective hydrosilations. I. The hydrosilation ofa,vdihydrogen functional oligopolydimethylsiloxanes with 3-vinyl-7-oxabicyclo[ 4.1.0]heptanes, J. Polym. Sci. A 31 (1993) 2563-2572. |
| [25] | D.J. David, H.B. Staley, Analytical Chemistry of the Polyurethanes, vol. 16, Wiley-Interscience, New York, 1969. |
| [26] | F.M. Li, S.J. Chen, Z.I. Li, J. Qiu, Vinyl monomers bearing chromophore moieties and their polymers. 1. Initiation and photochemical behavior of N-acryloyl-N-phenylpiperazines and their polymers, J. Polym. Sci. A 34 (1996) 1881-1888. |
| [27] | X.H. Hu, X.Y. Zhang, J.B. Dai, Synthesis and characterization of a novel waterborne stilbene-based polyurethane fluorescent brightener, Chin. Chem. Lett. 22 (2011) 997-1000. |
| [28] | N.Z. Galunov, B.M. Krasovitskii, O.N. Lyubenko, et al., Spectral properties and applications of the new 7H-benzo[de]pyrazolo[5,1-a]isoquinolin-7-ones, J. Lumin. 102 (2003) 119-124. |
| [29] | J. Qiu, Z.C. Li, Q.Y. Gao, et al., Vinyl monomers bearing chromophore moieties and their polymers. 3. Synthesis and photochemical behavior of acrylic monomers having phenothiazine moieties and their polymers, J. Polym. Sci. A 34 (1996) 3015-3023. |
| [30] | J. McCall, C. Alexander, M.M. Richter, Quenching of electrogenerated chemiluminescence by phenols, hydroquinones, catechols, and benzoquinones, Anal. Chem. 71 (1999) 2523-2527. |
| [31] | C. Turro, S.H. Bossmann, Y. Jenkins, J.K. Barton, N.J. Turro, Proton transfer quenching of the MLCT excited state of Ru(phen)2dppz2+ in homogeneous solution and bound to DNA, J. Am. Chem. Soc. 117 (1995) 9026-9032. |
| [32] | L. Biczok, H. Linschitz, Concerted electron and proton movement in quenching of triplet C60 and tetracene fluorescence by hydrogen-bonded phenol-base pairs, J. Phys. Chem. 99 (1995) 1843-1845. |
| [33] | G. Camino, S.M. Lomakin, M. Lazzari, Polydimethylsiloxane thermal degradation. Part 1. Kinetic aspects, Polymer 42 (2001) 2395-2402. |
| [34] | G. Deshpande, M.E. Rezac, The effect of phenyl content on the degradation of poly (dimethyl diphenyl) siloxane copolymers, Polym. Degrad. Stabil. 74 (2001) 363- 370. |
| [35] | P. Krol, K. Pielichowska, L. Byczynski, Thermal degradation kinetics of polyurethane- siloxane anionomers, Thermochim. Acta 507 (2010) 91-98. |
| [36] | W.J. Zhou, H. Yang, X.Z. Guo, J.J. Lu, Thermal degradation behaviors of some branched and linear polysiloxanes, Polym. Degrad. Stabil. 91 (2006) 1471-1475. |
| [37] | AATCC Test Method 140-2001, Dye and pigment migration in a pad-dry process, in: AATCC Technical Manual, American Association of Textile Chemists and Colorists, 2006. |