b Chengdu No. 1 Pharmaceutical Group Co., Ltd., Chengdu 610031, China;
c University of Chinese Academy of Sciences, Beijing 100049, China;
d Key Laboratory of Molecular Medicine, Ministry of Education, and Department of Biochemistry and Molecular Biology, Fudan University Shanghai Medical College, Shanghai 200032, China
Leonurus japonicus, widely distributed in China, is an important folk medicine for the treatment of gynecological and obstetrical diseases, commonly known as ‘‘Motherwort’’ or ‘‘I-Mu Ts’ao’’ around the world and ‘‘Yi-Mu-Cao’’ in China [1,2]. The phyto- chemical investigation on this species has afforded numerous natural compounds with various structural patterns, such as alkaloids, iridoids, diterpenoids, triterpenoids, flavonoids, glyco- sides, and other compounds [3]. Among these compounds, labdane-type diterpenoids are major constituents of the species L. japonicus, some of which exhibit anti-platelet aggregation [4], anti-cholinesterase [5] and anti-inflammatory activities [6]. However, no clerodane-type diterpenoid has been isolated from this plant. Interestingly, our current research on the chemical constituents of L. japonicus led to the isolation of a new clerodane diterpenoid, leojaponin A (1), with a C4–C7 oxa-bridge and a double bond between C2 and C3, and two new labdane diterpenoids, leojaponins B–C (2–3). Herein, we report the isolation, structural elucidation and cytotoxicity of compounds 1–3.
2. ExperimentalTheaerial parts of L. japonicus were collected in Xichang county, Sichuan Province,China, and identified by Prof. Xi-Wen Li. Voucher and specimens (KIB 20120601) were deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and isolation: The dried aerial parts of L. japonicus (15.0 kg) were extracted with 95% EtOH (3 × 40 L) at room temperature, and the combined solvents were evaporated in vacuo to yield a residue (1.5 kg). This residue was chromato- graphedonasilicagelcolumnelutingwithCHCl3–Me2CO(1:0,9:1, 8:2, 2:1, 1:1, 0:1) to afford fractions A–F. Fraction B (70.0 g) was subjected to MCI gel (90% MeOH–H2O) and chromatographed on silica gel (petroleum ether-Me2CO, 30:1–0:1) to afford subfrac- tions B1–B7. Fraction B2 (10.0 g) was subjected to RP-18 column chromatography (20–100% gradient MeOH–H2O) to afford sub- fractions B2.1–B2.9. Fraction B2.1 (0.03 g) was purified by semi- preparative HPLC (65% MeCN–H2O) to give compound 1 (12 mg); Fraction B2.2 (0.15 g) was repeatedly chromatographed on Sephadex LH-20, and finally by semi-preparative HPLC (55% MeCN–H2O) yielding compound 2 (14 mg); Fraction B2.3 (0.07 g) was purified repeatedly by LH-20 and semi-preparative HPLC (40% MeCN–H2O) to yield compound 3 (6 mg) (Fig. 1).
|
Download:
|
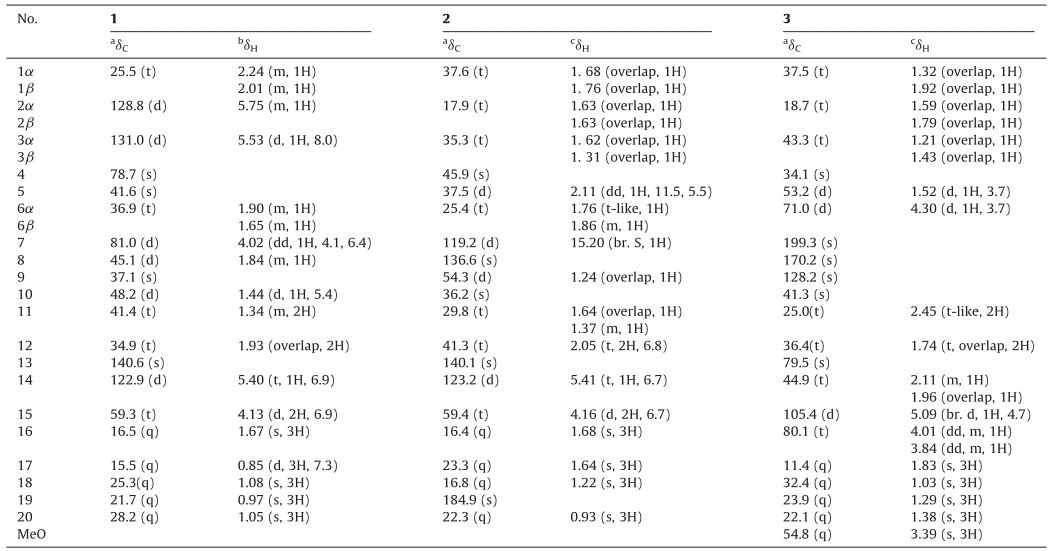
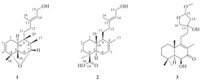
| Fig. 1. Structures of compounds 1–3. | |
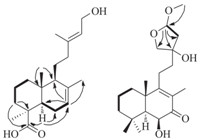
Leojaponin A(1) was obtainedasa colorlessoil, [α]19.0D -52.8 (c 0.21, MeOH) and UV (MeOH) λmax (log ε) 202 (3.65) nm. The molecular formula of 1 was assigned as C20H32O2 by HR-EI-MS(m/z 304.2402 [M]+, calcd. 304.2402) with five degrees of unsaturation. The IR spectrum indicated the presence of a hydroxyl group (3440 and 3426 cm-1). The 1H NMR spectrum displayed signals of one secondary methyl and four tertiary methyl groups. Three olefinic proton signals at δH 5.75 (m), 5.53 (d, J = 8.0 Hz) and 5.40 (t, J = 6.9 Hz) suggested the presence of two double bonds (Table 1). The 13C NMR and DEPT spectra exhibited signals for 20 carbons, including four quaternary carbons (including a single oxygenated one), six methines (including a single oxygenated one), five methylenes (including a single oxygenated one), and five methyls (Table 1). On the base of the HSQC spectrum, all protons were assigned unambiguously to their corresponding carbons. The above evidence implied that 1 was a clerodane diterpenoid, which was similar to the reported compound (3a, 4b, 13E)-4-ethox- yneoclerod-13-ene-3,15-diol [7]. The significant difference be- tween 1 and (3a, 4b,13E)-4-ethoxyneoclerod-13-ene-3,15-diol was that 1 possessed two more degrees of unsaturation than (3a, 4b,13E)-4-ethoxyneoclerod-13-ene-3,15-diol. One degree of unsa- turation was attributed to the double bond and the other one resulted from an additional ring based on the molecular formula. The location of the double bond was deduced to be between C-2 and C-3 by the HMBC correlations from H-10 to C-1, C-2; from H-1 to C-2, C-3 and the 1H – 1H COSY correlations of H-10/H-1/H-2/H-3. C-7 was deduced to be an oxygenated methine based on the chemical shifts of C-7 and H-7, and the 1H – 1H COSY of 1 supported the connectivities of H-8/H-7/H-6 (Fig. 2), which was confirmed by the HMBC correlations of H-8 with C-7 and C-6; of H-7 with C-6 and C-5 (Table 1). The location of the additional ring at C-4 and C-7 was determined by the HMBC correlations of H-7 with C-4, which suggested the connection of C-4 to C-7 through an oxygen bridge. The E geometry of the double bond between C-13 and C-14 was deduced from ROESY correlation of H-15 with Me-16 (Fig. 2).
|
Download:
|
Fig. 2. Key HMBC(H!C), 1H – 1H COSY(–)andROESY(H H)correlationsof1.
H)correlationsof1.
|
|
The relative stereochemistry of 1 was deduced by the ROESY data. H-10 was supposed to be a-oriented on the basis of its reported analogs [8]. ROESY correlations of H-10 with H-11 and H- 12, of H-1a with H-10 and H-12, of H-6a with H-11, suggested that Me-20 was b-oriented. ROESY correlations of H-1β with Me-20 and Me-18, of Me-19 with Me-18, of H-7 with Me-17, of H-17 with H-6β suggested that Me-18, Me-19, Me-17 and H-7 were all b- oriented (Fig. 2) (Figs. S1–10 in Supporting information).
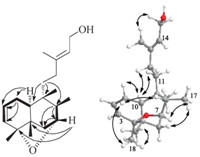
LeojaponinB(2)wasisolatedasacolorlessoil,[α]19.0D-68.2(c 0.16, MeOH) and UV (MeOH) λmax (log ε) 202 (3.99) nm. Its molecular formula was determined as C20H32O3by its HR-EI-MS (m/z 320.2344 [M]+, calcd. 320.2351), implying five degrees of unsaturation. The IR spectrum showed absorptions at 3449 and 1704 cm-1,revealingtheexistenceofhydroxylanδCarbonylgroups. The 1H NMRand 13C NMRspectra(Table1)of2werecomparedwith those of the known labdane-type diterpenoid, villenol, and showed close structural similarities [9]. Careful comparison of their NMR data suggested that the main differences resulted from the hydroxymethyl (C-19, δC 64.7) in villenol oxidized to a carboxyl (C-19,δC184.9)in2,whichwasconfirmedbytheHMBCcorrelations ofH-3anδH-5withC-19(Fig.3).ThedoublebondbetweenC-13and C-14 of 2 was E geometry based on the ROESY correlation of H-15 with Me-16. The relative configurations of 2 were established by analysis of its ROESY data. Considering the structures of labdane- type diterpenoids previously isolated from the species L. japonicus, Me-20 was supposed to beb-oriented [10]. The correlations of Me- 20 with H-11 and H-6β, of H-9 with H-5 and Me-18, and of H-6a with H-5 indicated that H-5, H-9 and Me-18 were all a-oriented (Figs. S11–20 in Supporting Information).
|
Download:
|
| Fig. 3. Selected HMBC (H!C) and 1H – 1H COSY (–) correlations of 2 and 3. | |
Leojaponin C (3), isolated as a colorless oil, [α]19.0 D +8.64 (c 0.22, MeOH), UV (MeOH) λmax (log ε) 253 (3.68) nm, gave the molecular formula, C2 1H 34O5, from its HR-EI-MS (m/z 366.2420 [M]+, calcd. 366.2406), requiring five degrees of unsaturation. The IR spectrum revealed the presence of hydroxyl groups (3441 cm-1) and double bonds (1646 cm-1). The 1H and 13C NMR data (Table 1) of 3 were highly similar to those reported for (6β)-15,16-epoxy- 15-ethoxy-6,13-dihydroxylabd-8-en-7-one [11], which was also one of labdane-type diterpenoids isolated in this plant. A careful comparison of their 1D NMR data, together with detailed HMBC and 1H – 1H COSY analysis indicated that the difference was due to an ethoxyl group (δC63.0, 15.2) in (6β)-15,16-epoxy-15-ethoxy- 6,13-dihydroxylabd-8-en-7-one replaced by a methoxyl group (δC 54.8) in 3. This was confirmed by HMBC correlations from MeO to C-15 (Fig. 3). In the ROESY spectrum, the correlations of Me-20 with Me-19 indicated that Me-19 was b-oriented. Correlations of Me-18 with H-5 and H-6 suggested that Me-18, H-5 and H-6 were on the same face as a-orientated (Figs. S21–30 in Supporting Information).
| Table 1 1H and 13C NMR data of compounds 1–3 in CDCl3(d in ppm, J in Hz). |
Compounds 1–3 were tested for their cytotoxicity against the HeLa cell line using the Alarmar-Blue assay [12]. All compounds were inactive with IC50values greater than 40mmol/L.
4. ConclusionThree new diterpenoids, leojaponins A–C (1–3), were isolated from L. japonicus for the first time. To our knowledge, leojaponin A (1) is the first example of clerodane diterpenoid obtained from L. japonicus. This investigation should provide valuable information for further understanding of the chemical constituents of L. japonicus.
AcknowledgmentsThis project was supported financially by the Natural Science Foundation of Yunnan Province (No. 2012FB178) and the project sponsored by SRF for ROCS, SEM to W.L. Xiao.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2014.01.047.
| [1] | The Editorial Board of Flora of China, Flora of China 65(2), Science Press, Beijing, 1977, pp. 505-511. |
| [2] | S.Y. Hu, A contribution to our knowledge of Leonurus L., I-mu-ts'ao, the Chinese motherwort, Am. J. Chin. Med. 4 (1976) 219-237. |
| [3] | L.H. Shen, S.L. Wang, Research progress on Leonurus Heterophyllus Sweet, Med. Plant 1 (2010) 48-51. |
| [4] | C.M. Lee, L.M. Jiang, H.S. Shang, et al., Prehispanolone, a novel platelet activating factor receptor antagonist from Leonurus heterophyllus, Br. J. Pharmacol. 103 (1991) 1719-1724. |
| [5] | T.M. Hung, T.C. Luan, B.T. Vinh, T.D. Cuong, B.S. Min, Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity, Phytother. Res. 25 (2011) 611-614. |
| [6] | S. Khan, O. Shehzad, H.G. Jin, et al., Anti-inflammatory mechanism of 15,16- epoxy-3 alpha-hydroxylabda-8,13(16), 14-trien-7-one via inhibition of LPS-induced multicellular signaling pathways, J. Nat. Prod. 75 (2012) 67-71. |
| [7] | S.M. Yang, S.H. Wu, X.D. Qin, X.D. Luo, D.G. Wu, Neoclerodane diterpenes from Amoora stellato-squamosa, Helv. Chim. Acta 87 (2004) 1279-1286. |
| [8] | S.F. Palmeira, L.M. Conserva, J.M. Barbosa, Clerodane diterpenes from Croton species: distribution and a compilation of their 13C NMR spectral data, Nat. Prod. Commun. 1 (2006) 319-344. |
| [9] | A.A. Hussein, M.J.J. Meyer, B. Rodríguez, Complete 1H and 13C NMR assignments of three labdane diterpenoids isolated from Leonotis ocymifolia and six other related compounds, Magn. Reson. Chem. 41 (2003) 147-151. |
| [10] | P.M. Giang, P.T. Son, K. Matsunami, H. Otsuka, New labdane-type diterpenoids from Leonurus heterophyllus Sw, Chem. Pharm. Bull. 53 (2005) 938-941. |
| [11] | H.Q. Gong, R. Wang, Y.P. Shi, New labdane-type diterpenoids from Leonurus heterophyllus, Helv. Chim. Acta 95 (2012) 618-625. |
| [12] | A. Schreer, C. Tinson, J.P. Sherry, K. Schirmer, Application of Alamar blue/5- carboxyfluorescein diacetate acetoxymethyl ester as a noninvasive cell viability assay in primary hepatocytes from rainbow trout, Anal. Biochem. 344 (2005) 76-85. |