b State Key Laboratory of Environmental and Biological Analysis, Department of Chemistry, Hong Kong Baptist University, Hong Kong Special Administrative Region, China
Fine particulate matter (PM2.5) with an aerodynamic diameter less than 2.5 mm mostly originates from coal combustion,vehicle exhaust and the open sources in Chinese cities [1]. The serious haze-fog episodes with the stronger PM2.5pollution during winter in China cities have recently caused big concern [2]. Epidemiologi- cal evidences show that there is a strong link between PM2.5 exposure and incidence or mortality of respiratory diseases including lung cancer [3, 4]. While the PM2.5 toxicity may be resulted from direct action of particle size,the toxicological effects are also mediated by chemical compositions present in PM2.5,such as polycyclic aromatic hydrocarbons (PAHs),nitrated PAHs (NPAHs),metals and water-soluble ions. Among them,PM2.5- bound PAHs and NPAHs are mainly released from the incomplete combustion of fossil fuel. NPAHs may also come from the photochemistry reaction of PAHs. Recently,PAHs have been demonstrated to be an important cause of the increased lung cancer in north China urban areas [5, 6]. NPAHs are particularly of great concern because they possess higher direct-acting mutage- nicity and carcinogenicity than the parent PAHs [7]. Thus,the pollution characteristics and health risks of PAHs and NPAHs on PM2.5are important for assessing PM2.5-induced toxicity.
Taiyuan,the capital city of Shanxi Province,is a center of coal- based electricity production and many chemicals industries. The city is currently facing serious problem of ambient PM2.5pollution. In this study,we investigated the pollution characteristics of ambient PM2.5-bound PAHs and NPAHs during wintertime in Taiyuan,China. The concentrations of PAHs and NPAHs were measured by using two gas chromatography-mass spectrometry (GC-MS) methods. Possible pollution sources and potential carcinogenicity of PM2.5were assessed.
2. ExperimentalConcurrent sampling of PM2.5aerosols on a rooftop of a 5-story building (about 25 m above ground) was conducted at a location in Taiyuan,China (Shanxi University,308150N,1128330E) from January 16 to January 23,2013. This site is located approximately 300 m away from a major roadway (Wucheng Road). There are no obvious industrial pollution sources around. Thus,the observa- tions could be typical of the general urban pollution in Taiyuan. PM2.5concentrations were measured using DustTrakTMII Aerosol Monitor (TSI Inc.,USA),while daily PM2.5samples were collected on quartz fiber filters (QFFs) for 24 h/day by using a PM2.5high volume air sampler (Thermo Anderson,USA),with a pump flow rate of 1.13 m3/min.
The standards of 16 PAHs including naphthalene (NAP),ace- naphthylene(ACY),acenaphthene(ACE),fluorine(FLO),phenantrene (PHE),anthracene (ANT),fluoranthene (FLA),pyrene (PYR),ben- zo[a]anthracene (BaA),chrysene (CHR),benzo[b] fluoranthene (BbF), benzo[k]fluoranthene (BkF),benzo[a]pyrene (BaP),indeno[1,2,3- cd]pyrene(IcdP),dibenzo[a, h]anthracene(DBahA),andbenzo[g, h, i]- perylene (BghiP) were obtained from Chem Service Inc. (West Chester,PA,USA). Deuterated PAH internal standard mixture solutions for PAH surrogates and the standards of three NPAHs [1- nitropyrene (1-NP),2-nitrofluorene (2-NFL),9-nitroanthracene (9- NA)] were obtained from AccuStandard Chem. Co. (New Haven,CT, USA).13C-labeled isotope internal standard mixture solutions for NPAH surrogates were purchased from Cambridge Isotope Labora- tories Inc. (Tewksbury,MA,USA). Internal and surrogate standards were added to all samples and calibration solutions for the quantitative analysis and quality control. PAHs and NPAHs on QFFs were extracted ultrasonically twice with dichloromethane (DCM). The extracts were concentrated by rotary evaporation to approxi- mately 1 mL and purified on a silica packed column. The PAHs and NPAHs fraction was eluted with 50 mL of DCM. The fraction was concentrated to 0.5 mL and analyzed by GC-MS. The PAH analysis wasconductedbyusingGC-MS(Agilent6890GCcoupledwith5975 MSD) in selected ion monitoring (SIM) mode. A HP-5MS capillary column (30 m × 0.25 mm × 0.25mm,Agilent,USA) was used. The oventemperaturewasheldat60 8C(1 min),andrampedat7 8C/min to 300 8C (5 min). The MSD was operated in electron impact ionization (EI) mode at 70 eV,and the ion source temperature was 230 8C. The analysis of NPAHs was conducted by using GC-MS (ThermoFischerScientificGCcoupledwithMSD)inSIMmodeunder negative chemical ionization (NCI). A TG-5SILMS capillary column (30 m × 0.25 mm × 0.25mm,Thermo,USA) was used. The oven temperature was programmed as 70 8C for 1 min and increased to 300 8C at a rate of 20 8C/min (6 min). The high-purity helium and methane were used as the carrier gas and chemical ionization reagent gas,respectively. The ion source temperature was 200 8C.
As for sample analysis and quality control,the solvent blanks and QFF blanks were analyzed with the real samples. Analysis of the two blanks was performed with every eight samples. The calibrations were examined with the analysis of standards for about every 20 samples. The quantification was performed by using the isotope dilution internal standard method. Method detection limits ranged from 1.1 ng/mL (PRY) to 10.4 ng/mL (DBahA) for PAHs and from 0.05 ng/mL (9-NA) to 2 ng/mL (1- NP) for NPAHs. The intra-day and inter-day relative standard deviations of the methods were less than 9%. Mean recoveries of 63%-128% for PAHs and 62%-98% for NPAHs were obtained for the entire analytical procedure.
The pollution characteristics and possible sources of PM2.5in duringwintertimeinTaiyuanwereassessedbyusingspecialindexes calculated by concentrations of PAHs andNPAHs on PM2.5according to the methods reported previously [8, 9, 10]. The method measuring PAH or NPAH ratio was applied to the source identification of coal combustion,vehicleexhaustandotheremissionsource.Forexample, the ratio of BaP/BghiP from 0.9 to 6.6 was referred to coal-burning pollution,while the value from 0.3 to 0.44 was for oil-burning pollution [8]. Likely,the ratios of PRY/BaP (<1.0),IcdP/BghiP (0.9), and 1-NP/PRY (0.001-0.36) were proposed as special indicators of coal combustion to atmospheric PAHs and NPAHs [9, 10]. The health risk for PM2.5was estimated by using the BaP equivalent (BaPeq) toxicity and individual carcinogenicity index according to the Potency Equivalency Factors (PEFs) of chemicals [10, 11].
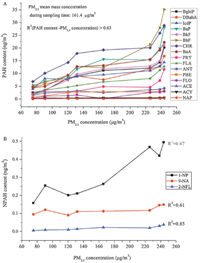
3. Results and discussionFrom the results depicted in Fig. 1,the 24-h mean mass concentration of PM2.5 during the sampling periods was 161.4 mg/m3,which was 2.1 times of the China National Ambient Quality Standard (NAAQS) for PM2.5 (75 mg/m3). The levels of PM2.5-bound PAHs or NPAHs elevated with the increase of PM2.5 concentration. In most cases,positive correlation was found between the concentrations of PM2.5and individual PAH or NPAH concentrations (R2values > 0.60),which indicated that PAHs and NPAHs might be important organic chemical markers on PM2.5 pollution in Taiyuan. The general trend of PAH concentration was CHR > BbF > BaP > BaA > BghiP ≈ BkF > IcdP ≈ DBahA > FLA > PYR > ANT > PHE > FLO > ACY > ACE > NAP (Table 1). Relatively, the levels of the 4-ring,5-ring and 6-ring PAHs,namely CHR,BbF, BaP,BaA,BghiP and BkF,were higher than the lower molecular weight PAHs with 2- or 3-rings. The averaged concentrations of each of the 16 PAHs bound to PM2.5ranged from 0.18 ng/m3to 17.9 ng/m3,which were close to the levels reported in Xiamen (3.04-12.49 ng/m3) and Beijing,China (0.28-9.0 ng/m3) as well as Gipuzkoa of Spain (0.3-8.29 ng/m3) [12, 13, 14]. The PAH levels detected inthis study were higher thanthose reported inEuropean and the US cities,for instance,Atlanta in the US (0.38-6.85 ng/m3) and Sa˜o Paulo of Brazil (0.01-5.21 ng/m3) [15, 16]. However,the levels were lower than those reported from other cities,such as Shenyang (3.1-166.1 ng/m3),Harbin (6.3-340 ng/m3) and Urumqi (0.11-1158 ng/m3) of China,as well as Coimbatore city in India (4.1-1632.3 ng/m3) and Seoul in South Korea (3.9-119.9 ng/m3) [17, 18, 19]. The averaged NPAH concentrations observed in this study (0.309 ng/m3for 1-NP; 0.118 ng/m3for 9-NA and 0.019 ng/m3for 2-NFL) were similar to those reported in Shenyang (0.434 ng/m3 for 1-NP) and the Marseilles area in France (0.061 ng/m3for 1-NP; 0.107 ng/m3for 9-NA and 0.021 ng/m3for 2-NFL) [17, 8],while higher than those in Copenhagen in Denmark (0.008-0.127 ng/m3 forNPAHs),AthensinGreece(0.02-0.06 ng/m3forNPAHs),andLos Angeles in the US (0.013 ng/m3for 1-NP; 0.013 ng/m3for 9-NA) [17]. Although significant difference in the concentrations of PM2.5-bound PAHs and NPAHs exists among different countries or cities,the concentrations in the developing countries were generally higher than the developed countries.
|
Download:
|
| Fig. 1. PM2.5-bound PAH (A) and NPAH (B) levels changed with PM2.5 mass concentrations during winter sampling periods in Taiyuan. | |
Local pollution sources can be important contributors on high PM2.5 level exposure in Taiyuan. For instance,there are some industrialfactoriessuchasTaiyuanPowerPlants,theIronandSteel Group Company,Xishan Coal and Power Group Company Ltd.,and Heavy Machinery Making Group Company Ltd. in the northwest of the city. In addition,households still use raw coal or briquettes for heating and cooking in some areas. These sources are likely to have large effects on PM2.5formation. On the other hand,Taiyuan is surrounded with mountains in three sides,which is prone to form topographic inversion. In winter,Taiyuan is often dominated by cold high-pressure system with low surface wind speeds, sometimes also accompanied by surface radiation inversion. These conditions are unfavorable for the dispersion of pollutants like fine particulates. Thus,Taiyuan has been found to be contaminated by relatively high levels of PM2.5,with contamination of PAHs and their derivatives. Ambient pollution of PAHs and NPAHs can be attributed to the coal combustion and vehicle exhaust. The possible sources of PM2.5pollution have been investigated with special diagnostic ratios of individual PAH and NPAH concentra- tions (such as BaP/BghiP,PRY/BaP,IcdP/BghiP and 1-NP/PRY concentration ratio) [8, 9, 10]. The data obtained from our study showed the ratio values of BaP/BghiP,PRY/BaP,IcdP/BghiP and 1- NP/PRY as 1.36,0.36,0.94 and 0.06,respectively (Table 1), suggesting that coal combustion systems,such as coal-fired power plant and industrial coal boilers,might be the sources of PAHs- and NPAHs-associating particulates in Taiyuan.
| Table 1 Daily mean levels of PAHs and NPAHs in PM2.5and special diagnostic concentration ratios of individual PAH and NPAH. |
The BaPeq values of PM2.5 containing PAHs and NPAHs were calculated based on PEFs of the individual compounds for risk assessment with the comparison to the national standard of 10 ng/m3 in China. The individual carcinogenicity index was estimated by the BaPeq values of the individual compounds compared to the standard limit of 1.0 × 10-5. It was reported previously that the chemicals might be a serious threat to human health when the individual carcinogenicity index was more than 1.0 × 10-5[11]. The data from our study showed that the BaPeq concentration of the ambient PM2.5-bound PAHs and NPAHs was 28.632 ng/m3 and the individual carcinogenicity index was 3.14 × 10-5(Table 2),implying that the PM2.5in Taiyuan might have potential cancer risk. The toxicity was mainly contributed from the PAHs pollution in the PM2.5 samples because only 3 NPAHs were quantified due to the limitation of the available standards. The obtained BaPeq values of PAHs (28.6 ng/m3) agreed well with the previously reported BaPeq concentration of PAHs in Taiyuan in 2009 and 2010 [6]. To the best of our knowledge,this is thefirst reportonNPAHs inPM2.5samples inTaiyuan,althoughthe BaPeq value (0.032 ng/m3) and the individual carcinogenicity index(3.55 × 10-8) fromthe NPAHswere muchsmallerthanthose of the PAHs (28.6 ng/m3and 3.14 × 10-5). Because NPAHs are more serious potential mutagens and carcinogens,the assessment on NPAHs risk to human health should be further investigated.
| Table 2 Estimated BaPeq concentrations and individual carcinogenicity index of PM2.5- bound PAHs and NPAHs in the samples collected in Taiyuan. |
Ambient PM2.5pollution was found serious during winter time in Taiyuan. The main source of PM2.5pollution appeared to be coal combustion. The 4-ring,5-ring and 6-ring PAHs were the predominant congeners on the PM2.5. The levels of CHR,BbF, BaP,BaA,BghiP and BkF were obviously higher than those of the Chinese national standard of PAHs. From the three measured NPAHs,1-NP was primary followed by 9-NA and 2-NFL. Notably, the obtained data of BaPeq toxicity and individual carcinogenicity index indicated that the PM2.5pollution in Taiyuan might post carcinogenic potential to human health. Hence,it is important to control regional combustion sources in order to reduce the PM2.5 pollution in Taiyuan.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21177078,21175086,21175025 and 41271531),Research Project Supported by Shanxi Scholarship Council of China (No. 2013-16),and 100 talents program of Shanxi Province.
| [1] | Y. You, Z. Bai, Research advances in exposure to ambient particulate matter and health effects, Asian J. Ecotoxicol. 7 (2012) 123-132. |
| [2] | M. Wang, M. Shao, S.H. Lu, et al., Evidence of coal combustion contribution to ambient VOCs during winter in Beijing, Chin. Chem. Lett. 24 (2013) 829-832. |
| [3] | P. Li, J. Xin, Y. Wang, et al., The acute effects of fine particles on respiratory mortality and morbidity in Beijing, 2004-2009, Environ. Sci. Pollut. Res. Int. 20 (2013) 6433-6444. |
| [4] | L.C. Vinikoor-Imler, J.A. Davis, T.J. Luben, An ecologic analysis of county-level PM2.5 concentrations and lung cancer incidence and mortality, Int. J. Environ. Res. Public Health 8 (2011) 1865-1871. |
| [5] | Y.X. Zhang, S. Tao, H.Z. Shen, et al., Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population, Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 21063-21067. |
| [6] | Z. Xia, X. Duan, S. Tao, et al., Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China, Environ. Pollut. 173 (2013) 150-156. |
| [7] | W. Wang, N. Jariyasopit, J. Schrlau, et al., Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games, Environ. Sci. Technol. 45 (2011) 6887-6895. |
| [8] | A. Albinet, E. Leoz-Garziandia, H. Budzinski, et al., Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): concentrations and sources, Sci. Total Environ. 384 (2007) 280-292. |
| [9] | N. Tang, T. Hattori, R. Taga, et al., Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in urban air particulates and their relationship to emission sources in the Pan-Japan Sea countries, Atmos. Environ. 39 (2005) 5817-5826. |
| [10] | D. Yang, S. Lan, Y. Ma, et al., Study of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in atmospheric particles in Dongguan, Environ. Chem. 31 (2012) 791-795. |
| [11] | J.F. Collins, J.P. Brown, G.V. Alexeeff, et al., Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives, Regul. Toxicol. Pharmacol. 28 (1998) 45-54. |
| [12] | L. Yang, Y. Wu, X. Zheng, et al., Characteristics of PM2.5-bound PAHs near a major road in Beijing during the 29th Olympic Game, Acta Sci. Circum. 31 (2011) 1144- 1153. |
| [13] | C.X. Ye, X.H. Wang, H.L. Yin, et al., Daily variation of priority polycyclic aromatic hydrocarbons in the atmospheric aerosol (PM2.5) of Xiamen city, Environ. Chem. 25 (2006) 356-360. |
| [14] | M. Villar-Vidal, A. Lertxundi, M.D. Martinez López de Dicastillo, et al., Air polycyclic aromatic hydrocarbons (PAHs) associated with PM2.5 in a North Cantabric coast urban environment, Chemosphere 99 (2014) 233-238. |
| [15] | Z. Li, E.N. Porter, A. Sjödin, et al., Characterization of PM2.5-bound polycyclic aromatic hydrocarbons in Atlanta - seasonal variations at urban, suburban, and rural ambient air monitoring sites, Atmos. Environ. 43 (2009) 4187-4193. |
| [16] | C. Bourottea, M.C. Fortic, S. Taniguchid, et al., A wintertime study of PAHs in fine and coarse aerosols in Sáo Paulo city, Brazil, Atmos. Environ. 39 (2005) 3799- 3811. |
| [17] | J. Zhan, Y. Yang, D.J. Liu, et al., The research progress of nitrated polycyclic aromatic hydrocarbons in the atmosphere, Sci. Sin. Terrae 42 (2012) 1-9. |
| [18] | S.S. Park, Y.J. Kim, C.H. Kang, Polycyclic aromatic hydrocarbons in bulk PM2.5 and size-segregated aerosol particle samples measured in an urban environment, Environ. Monit. Assess. 128 (2007) 231-240. |
| [19] | R. Mohanraj, S. Dhanakumar, G. Solaraj, Polycyclic aromatic hydrocarbons bound to PM2.5 in urban Coimbatore, India with emphasis on source apportionment, Sci. World J. (2012) 980843. |






