b Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz 51664, Iran
1. Introduction
Graphene,a novel material consisting of a monolayer of honeycomb-like crystal lattice of sp2-hybridized carbon atoms,has today become one of the hot spots of nanotechnology-oriented material science. Due to its 2D structure,graphene has versatile properties,such as high electron mobility,large surface area and robust mechanical strength. Graphene provides the greatest sensing area per unit volume because each atom of graphene is a surface atom [1]. Therefore,electron transport through graphene is highly dependent on electron donating or accepting molecules adsorbed on its surface,rendering graphene based materials as excellent candidates used in room temperature chemiresistor-type sensing applications [2]. Single graphene flakes have been produced with the traditional micromechanical cleaving technique [3] and thermal or plasma enhanced chemical vapor deposition methods [4, 5]. However,the production of high-quality,singlelayered graphene sheets as well as their deposition to a specific spot on a substrate is still challenging tasks,at least on a large scale. Graphene oxide (GO),on the other hand,can be produced on a large scale and processed in solution [6, 7, 8]. GO,as a basic material for the preparation of individual graphene sheets in bulk-quantity, has attracted great attention in recent years Additionally,the incredibly large specific surface area (two accessible sides),the abundant oxygen containing surface functionalities,such as epoxide,hydroxyl,and carboxylic acid groups,and the high water solubility afford GO sheets great promise for many more applications [6, 7, 8]. Its amazing characteristics,include a large surface area,extraordinary electrical [9, 10, 11],thermal [12], mechanical [13],and structural properties [14a, d],make graphene a highly versatile carbon species with promising applications in composites [15a, d],transparent conducting films [1, 16],sensors [17],supercapacitors [18a and b],nanoelectronics [19],batteries [20a, d],catalyst supports [21],and biotechnology [22].
On the other hand,polymer composites are valued as strong, durable,and multifunctional species with potential applications as high performance materials. Yet,the cost of nanoparticles,their availability and the challenges that remain to achieve good dispersions pose major obstacles in their production [23]. One way to produce high performance composites is filling polymers with nanotubes [24]. A better alternative is dispersing of just a small amount of graphene in polymers which often turns them into tough,lightweight composites with good electrical conductivity and thermal stability. However,this option requires production of graphene sheets on an acceptable scale followed by their homogeneous incorporation into the polymers [25].
Polystyrene (PS) is one of the most widely used commercial polymers. The integration of GO in a PS matrix produced nanocomposite thin films that are semiconducting and exhibit an ambipolar field effect [25]. The dispersion of graphene into a PS matrix has stimulated considerable research,including the preparation of graphene-PS composite thin films by adding linear mono-dispersed PS functionalized GO dispersion in DMF by Eda et al. [25]. Furthermore,there are some studies on the preparation of PS-graphene nanocomposite by polymerization where styrene monomer is dispersed in the water phase and polymerized with a water soluble radical initiator in the presence of GO. For example, fabrication of PS/graphene nanocomposite by microemulsion polymerization was reported by Ahn et al. [26]. Also,Ding et al. [27] synthesized the PS intercalated GO nanocomposite via polymerization in the presence of sodium laurel sulfate as emulsifier. To conclude,Hu et al. [28] prepared the graphene nanosheets-PS nanocomposites by in situ polymerization and reduction of GO using hydrazine hydrate.
In order to upgrade the properties of styrene and reach the desired PS/GO interaction,we decided to synthesize GO incorporated into PS. After the nanocomposite has been synthesized,we used PS/GONC for studying the electrochemical behavior of histamine. To the best of our knowledge,this is the first report of the determination of histamine based on its direct electrochemical oxidation by a PS/GONC modified glassy carbon electrode (PS/ GONC/GCE). Using this system,detection of sensitive quantities of histamine was realized using differential pulse voltammetric method. The electrode has an ultra low detection limit of 0.03 mmol/L during histamine electrooxidation,as compared with previous reports (Table 1) [29, 30, 31, 32, 33, 34]. The excellent capacity of the electrode for histamine electrooxidation has demonstrated its excellent performance. Furthermore,the proposed polymeric sensor (PS/GONC/GCE) was successfully used to detect histamine in fish samples.
| Table 1 Analytical parameters for detection of histamine at several electrochemical sensors. |
Electrochemical experiments were performed with a computercontrolled Autolab modular electrochemical system (Eco Chemie Ultecht,The Netherlands),drivenwith GPES software (Eco Chemie). A conventional three-electrode cell was used with an Ag/AgCl (Methrom,TheNetherlands) as a reference electrode and a Ptwire as a counter-electrode. The working electrode was PS/GONC/GCE. All measurements were conducted at a thermostated temperature of 20 ± 1 ℃. The transmission electron microscope (TEM) images were obtained at JEOL-1200 EXTEM(Japan). The images of scanning electron microscope (SEM) were obtained at Hitachi S-4800 (Japan). 2.2. Chemical and reagents
All chemicals used were analytical grade from Merck (Darmstadt, Germany) and were used without further purification. Briton Robinson buffers were prepared by initially dissolving 10 mL concentrated orthophosphoric acid,8.7 mL glacial acetic acid and 9.27 g boric acid in water and diluting to 1.0 L in a volumetric flask. This solution was subsequently used to prepare appropriate buffers by addition of 7.5 mol/L NaOH to reach the desired pH value. Voltammetric experiments of histamine were carried out in 0.15 mol/L Briton Robinson buffer of pH 4.00. Histamine was purchased as hydrochloride salts from Sigma (USA). Dansyl chloride for dansylation was obtained from Sigma-Aldrich (Switzerland).
Stock solutions of the histamine were prepared separately to concentrations of 1 mg/mL in 0.1 mol/L hydrochloric acid (HCl). A working solution was prepared by diluting 1 mL of each stock solution in 0.1 mol/L HCl to a final volume of 10 mL. The Dansyl chloride solution (5 mg/mL) was prepared by dissolving 500 mg of Dansyl chloride in 100 ml of acetone. All solutions were kept at a temperature of 4 ℃ prior to use.
Histamine extraction from the samples was carried out according to the procedures developed by Mah et al. [35] with a little modification. A 3 g slurry of each sample was transferred into a centrifuge tube containing 10 mL of 5% (w/v) trichloroacetic acid (TCA) and 200 μL of 1,7-diaminoheptane. The mixture was vortexed for 15 min and then centrifuged at 5000 × g for 10 min at 4 ℃. The supernatant was collected and the residue was extracted again with the same volume of TCA. Both supernatants were filtered through Whatman paper No. 1 and combined. The final volume was adjusted to 25 mL with TCA. 2.3. Synthesis of the PS/GONC
Initially,the GO was prepared according to our previous works [36, 37]. In brief,pure graphite (1.0 g) and potassiumchlorate (8.0 g) were mixed in fuming nitric acid (20 mL) at room temperature without subsequent aging and stirred for 24 h. The following processes,such aswashing,filtration and cleaning,were carried out as in theBrodiemethod. Thesynthesizedgraphiteoxide (10 mg)was dispersed in aqueous NaOHsolution (30 mL) at pH 10. The prepared graphite oxide was sonicated by a bath type sonicator (Sonoswiss SW3-H,38 kHz,Switzerland) for 1 h. After ultrasonication,samples were immediately precipitated by a centrifuge at 15,000 rpm for 10 min. The graphene oxide sheets were extracted carefully.
Then,1.5 mL octanol was added to an aqueous solution containing 75 mL distilled water and 250 mg sodium dodecyl sulfate. Then 50 mg of GO was added. The mixture was sonicated for 1 h minimum and transferred to a three necked round bottom flask (rbf),cooled in an ice bath and equipped with a condenser, dripping funnel,and nitrogen inlet. Then a mixture of 2.5 g styrene and 0.025 g benzoyl peroxide was added under a stream of N2 in 30 min. The resulting mixture was homogenized by sonication at 0 ℃ for another 30 min. Then polymerization was carried out by increasing the temperature of the mixture in an oil bath to 80 ℃ for 4 h under an inert nitrogen blanket. Polymerization rendered a stable gray colored PS/GO emulsion from which excess surfactants and residual monomers were washed away by methanol and water giving a precipitate that was dried at 60 ℃ for 24 h and used for further characterizations. PS/GO films were prepared by dissolving 25 mg of GO powder in 100 mL of dichloromethane. The solution was then cast on a glass plate and dried at room temperature to form the free standing and uniform film. 2.4. Characterization of PS/GONC
The general morphologies of synthesized PS/GONC were observed by SEM and shown in Fig. 1(a and b). A flat surface was shown in Fig. 1,which demonstrates a single atomic thickness layer as the structural feature. The properties of PS/GONC are highly related to their micro structures,their dispersity and the morphology of GO. The morphology of PS/GONC was examined by TEM (Fig. 1c and d),where the image of the prepared composite showed GO nanosheets distributed over the PS.

|
Download:
|
| Fig. 1.SEM images GO and PS/GONC/GCE (a and b). TEM image of GO and PS/GONC/GCE (c and d). | |
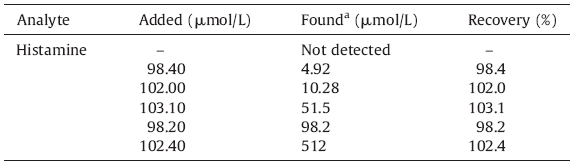
The electrochemical activity of PS/GONC/GCE was examined by cyclic voltammetry. Fig. 2A shows the cyclic voltammograms responses of 0.5 mmol/L histamine at PS/GONC/GCE in (pH 7.4) at scan rate 100 mV/s. The PS/GONC/GCE exhibits significant oxidation currents starting around 0.65 V vs. Ag/AgCl and no reduction signal observed in the reversed scan. In contrast,low redox activity is observed at the PS/GONC in the absence of histamine over the same potential range. A substantial negative shift of the anodic peak potential and dramatic increase of current indicate a significant ability of PS/GONC/GCE to oxidize histamine. Due to high specific area of PS/GONC/GCE,histamine penetrates through the GO layer leading to higher sensitivity of the sensor. The reproducibility of PS/GONC/GCE for electrooxidation of histamine is evaluated by four independently prepared nanocomposites. Then cyclic voltammograms are recorded in 0.5 mmol/L histamine solution (RSD was 4.00%). The relative standard deviation (RSD) of the peak currents of 0.5 mmol/L histamine for ten repeat determinations is also 3.30%. Then at the surface of PS/GONC/ GCE not only the over-voltage for histamine oxidation decreased, but the antifouling properties of the film also improved reproducibility. In order to optimize the electrocatalytic response of PS/GONC/GCE toward histamine oxidation,the effect of pH on the catalytic oxidation behavior was investigated. The DPV of PS/ GONC/GCE in 0.1 mmol/L histamine at different pH values (2-11) was recorded. With increasing pH values the histamine oxidation peak potential shifts to less positive value while the peak current is almost unchanged. Since more reproducible results and high electroactivity of nanocomposite are observed at pH 4-10,the histamine measurements were acquired at pH 5. The differential pulse voltammograms of the modified electrode in the presence different concentrations of histamine,0.1-3 mmol/L were recorded (Fig. 2B). A linear dependence of the catalytic currents vs. concentration of histamine can be fitted in the equation I (nA) = 0.5133 histamine μA/μM + 0.332 μA and R = 0.9923. The detection limit was 0.03 mmol/L at a signal to noise ratio of 3 (Fig. 2C). Oxidation mechanisms for the histamine acids are presented in Scheme 1.
|
Download:
|
| Fig. 2.(A) Cyclic voltammograms of PS/GONC/GCE in pH 5.0 solution at scan rate 100 mV/s in the absence (a) and presence of 0.5 mmol/L histamine (b). (B) DPV of PS/GONC/ GCE in pH 5.0 buffer containing different concentration of histamine, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 1.5, 2, 2.5 and 3 μmol/L. (C) Plot of peak current vs. histamine concentration. | |
|
Download:
|
| Scheme 1.Oxidation mechanism of histamine on PS/GONC/GCE. | |
The recovery of the analytes was measured by spiked histamine into diluted fish samples (Table 2). The DPVs were recorded after the fish samples were spiked with various amounts of histamine within the working concentration range. Recoveries were found to lie in range of 98.2%-103.1%. Good recoveries of histamine were achieved by this method,meaning the application of the proposed sensor to the analysis of histamine in fish samples could be easily assessed.
| Table 2 Recoveries of histamine from fish samples with PS/GONC/GCE. |
In summary,a polystyrene-graphene oxide nanocomposite (PS/ GONC) was synthesized using in situ polymerization. The results show that the facile and environmental-friendly technique presented here is an effective and promising method of functionalization of GO by PS. For the first time,the electrooxidation of histamine was successfully performed using the PS/GONC/GCE. The prepared nansosensorshowsexcellentelectroactivity for histamineoxidation. The analytical performance of PS/GONC/GCE indicates that it can be used as a sensitive electrochemical detector for sub-micromolar detection of histamine when coupled with a flow system.
AcknowledgmentsWe gratefully acknowledge the support of this work by Drug Applied Research Center,Tabriz University of Medical Science and Payame Noor University.
| [1] | F. Schedin, A.K. Geim, S.V. Morozov, et al., Detection of individual gas molecules adsorbed on graphene, Nat. Mater. 6 (2007) 652.655. |
| [2] | F. Yavari, N. Koratkar, Graphene-based chemical sensors, J. Phys. Chem. Lett. 3 (2012) 1746.1753. |
| [3] | E. Massera, V. La Ferrara, M. Miglietta, et al., Gas sensors based on graphene: comparison of two different fabrication approaches, Chim. Oggi. 29 (2011) 39.41. |
| [4] | A. Reina, X.T. Jia, J. Ho, et al., Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition, Nano Lett. 9 (2009) 30.35. |
| [5] | M.Y. Zhu, J.J. Wang, B.C. Holloway, et al., A mechanism for carbon nanosheet formation, Carbon 45 (2007) 2229.2234. |
| [6] | D. Li, M.B. MÜller, S. Gilje, R.B. Kaner, G.G. Wallance, Processable aqueous dispersions of graphene nanosheets, Nat. Nanotechnol. 3 (2008) 101.105. |
| [7] | S. Park, R.S. Ruoff, Chemical methods for the production of graphemes, Nat. Nanotechnol. 4 (2009) 217.224. |
| [8] | V.C. Tung, M.J. Allen, Y. Yang, R.B. Kaner, High-throughput solution processing of large-scale grapheme, Nat. Nanotechnol. 4 (2009) 25.29. |
| [9] | K.S. Novoselov, A.K. Geim, S.V. Morozov, et al., Electric field effect in atomically thin carbon films, Science 306 (2004) 666.669. |
| [10] | http://www.nobelprize.org/nobel_prizes/physics/laureates/2010/advanced-physicsprize2010.pdf.. |
| [11] | P. Avouris, Z.H. Chen, V. Perebeinos, Carbon-based electronics, Nat. Nanotechnol. 2 (2007) 605.615. |
| [12] | A.A. Balandin, S. Ghosh, W.Z. Bao, et al., Superior thermal conductivity of singlelayer graphene, Nano Lett. 8 (2008) 902.907. |
| [13] | T.J. Booth, P. Blake, R.R. Nair, et al., Macroscopic graphene membranes and their extraordinary stiffness, Nano Lett. 8 (2008) 2442.2446. |
| [14] | (a) C.G. Lee, X.D. Wei, J.W. Kysar, J. Hone, Measurement of the elastic properties and intrinsic strength of monolayer graphene, Science 321 (2008) 385.388; (b) X.L. Wu, P. Liu, Facile preparation and characterization of graphene nanosheets/polystyrene composites, Macromol. Res. 18 (2010) 1008.1012; (c) B.Z. Jang, A. Zhamu, Processing of nanographene platelets (NGPs) and NGP nanocomposites: a review, J. Mater. Sci. 43 (2008) 5092.5101; (d) S. Stankovich, D.A. Dikin, G.H.B. Dommett, et al., Graphene-based composite materials, Nature 442 (2006) 282.286; (e) T. Ramanathan, A.A. Abdala, S. Stankovich, et al., Functionalized graphene sheets for polymer nanocomposites, Nat. Nanotechnol. 3 (2008) 327.331. |
| [15] | (a) R. Sengupta, M. Bhattacharya, S. Bandyopadhyay, A.K. Bhowmick, A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites, Prog. Polym. Sci. 36 (2011) 638.670; (b) H.A. Becerril, J. Mao, Z.F. Liu, et al., Evaluation of solution-processed reduced graphene oxide films as transparent conductors, ACS Nano 2 (2008) 463.470; (c) K.S. Kim, Y. Zhao, H. Jang, et al., Large-scale pattern growth of graphene films for stretchable transparent electrodes, Nature 457 (2009) 706.710; (d) X.L. Li, G.Y. Zhang, X.D. Bai, et al., Highly conducting graphene sheets and Langmuir.Blodgett films, Nat. Nanotechnol. 3 (2008) 538.542. |
| [16] | X. Wang, L.J. Zhi, N. Tsao, et al., Transparent carbon films as electrodes in organic solar cells, Angew. Chem. Int. Ed. 47 (2008) 2990.2992. |
| [17] | J.T. Robinson, F.K. Perkins, E.S. Snow, Z.Q. Wei, P.E. Sheehan, Reduced graphene oxide molecular sensors, Nano Lett. 8 (2008) 3137.3140. |
| [18] | (a) M.D. Stoller, S.J. Park, Y.W. Zhu, J.H. An, R.S. Ruoff, Graphene-based ultracapacitors, Nano Lett. 8 (2008) 3498.3502; (b) A. Das, S. Pisana, B. Chakraborty, et al., Monitoring dopants by raman scattering in an electrochemically top-gated graphene transistor, Nat. Nanotechnol. 3 (2008) 210.215. |
| [19] | G. Eda, G. Fanchini, M. Chhowalla, Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material, Nat. Nanotechnol. 3 (2008) 270.274. |
| [20] | (a) E. Yoo, J. Kim, E.Hosono, et al., Large reversible Li storage of graphene nanosheet families foruseinrechargeablelithiumionbatteries,NanoLett.8(2008)2277.2282; (b) P.K. Ang,W. Chen, A.T.S. Wee, K.P. Loh, Solution-gated epitaxial graphene as pH sensor, J. Am. Chem. Soc. 130 (2008) 14392.14393; (c) J. Dayen, A.Mahmood, D.S. Golubev, et al., Side-gated transport in focused-ionbeam- fabricated multilayered graphene nanoribbons, Small 4 (2008) 716.720; (d) Y.C. Si, E.T. Samulski, Exfoliated graphene separated by platinumnanoparticles, Chem. Mater. 20 (2008) 6792.6797. |
| [21] | Y.X. Xu, H. Bai, G.W. Lu, C. Li, G.Q. Shi, Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets, J. Am. Chem. Soc. 130 (2008) 5856.5857. |
| [22] | R. Muszynski, B. Seger, P.V. Kamat, Decorating graphene sheets with gold nanoparticles, J. Phys. Chem. C 112 (2008) 5263.5266. |
| [23] | J. Jordan, K.I. Jacob, R. Tannenbaum, M.A. Sharaf, I. Jasiuk, Experimental trends in polymer nanocomposites.a review, Mater. Sci. Eng. A 393 (2005) 1.11. |
| [24] | N.A. Kotov,Materials science: carbon sheet solutions, Nature 442 (2006) 254.255. |
| [25] | G. Eda, M. Chhowalla, Graphene-based composite thin films for electronics, Nano Lett. 9 (2009) 814.818. |
| [26] | A.S. Patole, S.P. Patole, H. Kang, et al., A facile approach to the fabrication of graphene/polystyrene nanocomposite by in situ microemulsion polymerization, J. Colloid Interface Sci. 350 (2010) 530.537. |
| [27] | R.F. Ding, Y. Hua, Z. Gui, et al., Preparation and characterization of polystyrene/ graphite oxide nanocomposite by emulsion polymerization, Polym. Degrad. Stab. 81 (2003) 473.476. |
| [28] | H.T. Hu, X.B. Wang, J.C. Wang, et al., Preparation and properties of graphene nanosheets-polystyrene nanocomposites via in situ emulsion polymerization, Chem. Phys. Lett. 484 (2010) 247.253. |
| [29] | S. Pé>rez, J. Bartrol., E. Fàbregas, Amperometric biosensor for the determination of histamine in fish samples, Food Chem. 141 (2013) 4066.4072. |
| [30] | D. Telsnig, K. Kalcher, A. Leitner, A. Ortner, Design of an amperometric biosensor for the determination of biogenic amines using screen printed carbon working electrodes, Electroanalysis 25 (2013) 47.50. |
| [31] | J. S.varc-Gajic, Z. Stojanovic, Electrocatalytic determination of histamine on a nickel-film glassy carbon electrode, Electroanalysis 22 (2010) 2931.2939. |
| [32] | C.M. Keow, F. Abu Bakar, A.B. Salleh, et al., Screen-printed histamine biosensors fabricated from the entrapment of diamine oxidase in a photocured poly (HEMA) film, Int. J. Electrochem. Sci. 7 (2012) 4702.4715. |
| [33] | M. Di Fusco, R. Federico, A. Boffi, et al., Characterization and application of a diamine oxidase from Lathyrus sativus as component of an electrochemical biosensor for the determination of biogenic amines in wine and beer, Anal. Bioanal. Chem. 401 (2011) 707.716. |
| [34] | R. Draisci, P.G. Volpe, O.L. Lucentini, et al., Determination of biogenic amines with an electrochemical biosensor and its application to salted anchovies, Food Chem. 62 (1998) 225.232. |
| [35] | H.K. Mah, J.H. Han, Y.J. Oh, M.G. Kim, H.J. Hwang, Biogenic amines in Jeotkals, Korean salted and fermented fish products, Food Chem. 79 (2002) 239.243. |
| [36] | M. Hasanzadeh, N. Shadjou, (Fe3O4)-Graphene oxide-SO3H as a new magnetic nanocatalyst for electro-oxidation and determination of selected parabens, J. Nanosci. Nanotechnol. 13 (2013) 4909.4916. |
| [37] | E. Omidinia, N. Shadjou, M. Hasanzadeh, (Fe3O4)-graphene oxide as a novel magnetic nanomaterial for non-enzymatic determination of phenylalanine, Mater. Sci. Eng. C 33 (2013) 4624.4632. |