b Department of Polymer, Science and Research Branch, Islamic Azad University, Yazd, Iran;
c Young Researchers Society, Shahid Bahonar University of Kerman, P.O. Box 76175-133, Kerman, Iran
1. Introduction
Presently,determination of trace heavy and precious metals in environmental samples is essential,because they have negative effects on human health [1, 2].
Copper is one of the most widely distributed elements in the environments of industrialized countries. It is present in all organisms,land and marine. It has been shown that copper is an essential element in many biological processes,such as blood formation and the function of many important enzymes. Copper is classified as a biogenic element,playing a significant role in photosynthesis,metabolism of nitrogen compounds,or regulation of RNA and DNA transcription process [3].
Silver is an industrially important element. The widespread use of silver compounds and silver-containing procedures in industry, medicine,jewelry,cloud seeding,and in the disinfection of drinking water has resulted in increasing silver content of environmental samples. It is used for the preparation of corrosion- resistant alloys,and its compounds are extensively used in the processing of foods,drugs,beverages,and in filters and other equipment to purify water [4].
The importance of palladium has grown in recent years due to its increasing use in the production of dental and medical devices, jewelry,and catalytic converters. Although the benefits of car catalysts are indisputable,the emission of Pd into the environment is largely associated with the production and recycling of catalytic converters in the metal finishing industry as well as the operation of vehicle catalysts [5].
All of these issues emphasize the importance of identifying and quantifying Cu,Ag,and Pd to provide comprehensive information about their properties and human healthrelevance. For thispurpose, several analytical methods have been developed to measure these ions in clinical,environmental,industrial,and pharmaceutical samples including: spectrophotometry,flame atomic absorption spectrometry (FAAS),inductively coupled plasma-mass spectrometry (ICP-MS),inductively coupled plasma-atomic emission spectrometry (ICP-AES),and electrochemical methods.
Electrothermal atomic absorption spectrometry (ETAAS) is a good technique for the determination of ultra-trace amounts of heavy metals in several types of samples due to its sensitivity [6]. But by reason of ultra-low concentration of metals and high concentration of interfering matrix components in most real samples,ETAAS often requires a suitable pretreatment step. Various techniques have been used for the separation and preconcentration of Cu,Ag,and Pd,such as dispersive liquid- liquid microextraction (DLLME),solidified floating organic drop microextraction (SFODME),and cloud point extraction (CPE).
To the best of our knowledge,there was no previous literature on the application of modified Al2O3 and ETAAS for simultaneous column preconcentration and determination of ultra-trace amounts of copper,silver,and palladium. In this work,the adsorption behavior of Cu,Ag,and Pd on modified Al2O3 with polyethylenimine (PEI) as a novel solid phase extractor was studied. Experimental parameters affecting the column preconcentration and determination of metals,such as ETAAS temperature program,pH,eluent type,sample and eluent flow rates, sample volume,and interfering ions were studied and optimized. The proposed method has been applied for the determination of trace amounts of Cu,Ag,and Pd in sea water,synthetic samples, and standard reference materials with satisfactory results. 2. Experimental 2.1. Apparatus
Copper,silver,and palladium measurements were performed with a Varian Spectra AA 220 atomic absorption spectrometer (Varian,Australia) with a deuterium lamp background correction and equipped with a graphite furnace (GTA-110 series). Optimum operating parameters for ETAAS are given in Table 1. A Metrohm 713 pH meter (Metrohm,Switzerland) was used for pH measurements with a combined glass calomel electrode. Thermogravimetric analysis (TGA) was accomplished with a Perkin-Elmer TGAUSA (pyris) (Perkin-Elmer,USA). Also a mechanical shaker with speed control was used for preparation of the adsorbent. A Thermo Finnigan Flash EA1112 microanalyzer (Finnigan,Germany) was used for determination of C,H,N percentage.
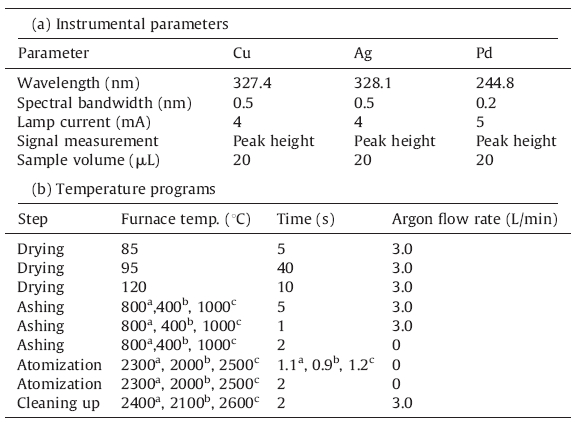
| Table 1 Instrumental parameters and thermal programs of ETAAS for determination of Cu, Ag and Pd. |
All chemicals were of analytical reagent grade,and deionized water was used in all experiments. Stock solutions of copper (1000 mg/L) and silver (1000 mg/L) were prepared by dissolving the appropriate amount of copper nitrate (Cu(NO3)2,Merck, Germany) and silver nitrate (AgNO3,Merck,Germany) in deionized water,respectively. A stock solution of Pd2+ (1000 mg/L) was prepared by dissolving a proper amount of palladium chloride (PdCl2,Merck,Germany) in 10.0 mL of HCl (1.0 mol/L) and diluted to 250.0 mL in a standard flask. Solutions of lower concentrations were prepared daily by a suitable dilution of the stock solutions with deionized water. Also,a solution of polyethylenimine (5%) (Molecular weight: 600,000-1,000,000) (Fluka,Switzerland) was prepared in deionized water. Al2O3 (Fluka,Switzerland) was used as an adsorbent. A 1.0 mol/L solution of thiourea (Merck,Germany) was prepared by dissolving a proper amount of thiourea in deionized water. Buffer solution was prepared from 0.1 mol/L of KH2PO4/K2HPO4 (Merck,Germany) for pH7. HCl,HNO3,and NaNO3 were purchased from Merck (Germany). 2.3. Preparation of modified adsorbent
The modified alumina with PEI (PEI/Al2O3) was prepared as following: 1.0 g of alumina,0.5 g of NaNO3,and 5.0 mL of PEI (5%) was mixed,and the pH was adjusted to about 7.0. The obtained suspension was shaken for 24 h. Then,the adsorbent was filtered, washed several times with deionized water and finally dried in a desiccator. It was characterized by elemental analysis (C,H,N) (Table 2) and TGA. The thermogravimetric analysis curve of the PEI/Al2O3 adsorbent shows four mass loss steps. The 1.7% mass loss up to 88.6 ℃ in the first step is due to adsorbed water. In the second step,the mass loss is 3.9% up to 296.4 ℃. In the third step,mass loss is 3.9% up to 506.5 ℃,and in the fourth step,mass loss is 1.53% up to 723.1 ℃. The mass losses in the second,third and fourth steps correspond with PEI.
| Table 2 Elemental analysis (C, H, N) for two kinds of adsorbent. |
A total of 50.0 mg of modified adsorbent (PEI/Al2O3) was slurred in water and then poured into a funnel-tipped glass tube (length: 80 mm,diameter: 5 mm). Before use,the column was conditioned with the buffer solution at pH 7.0. Cotton was placed at the bottom and the top of the column to allow the adsorbent to settle properly. 2.5. Recommended procedure
100.0 mL of the standard multi-element solution containing 100.0 ng of Cu,Ag,and Pd and 10.0 mL of buffer solution (pH = 7) was passed through the column with the desired flow rate (2.0 mL/ min). Then,the formed metal chelates that settled on the column were desorbed with two 5.0 mL of eluent solution at the flow rate of 4.0 mL/min. Each 5.0 mL of eluent contains 3.0 mL of thiourea (1.0 mol/L) and 2.0 mL of HCl (1.0 mol/L). Finally,20.0 μL of each 5.0 mL of eluate solution was automatically injected by the autosampler into the graphite tube and then the absorbance of Cu, Ag,and Pd were measured under the operating conditions summarized in Table 1. 2.6. Samples preparation 2.6.1. Sea water
The selected water sample (Caspian Sea water) was filtered through a Millipore filter to remove suspended particulate matter. Then,2 mL of nitric acid was added to prevent adsorption of the metallic ions onto the flask walls,and the solution was stored at 4 ℃ in a refrigerator,and the suggested method was applied to the determination of copper,silver,and palladium [7]. For preconcentration, the pH of the samples was adjusted to 7.0 before analyzing by the described procedure. 2.6.2. Standard reference material
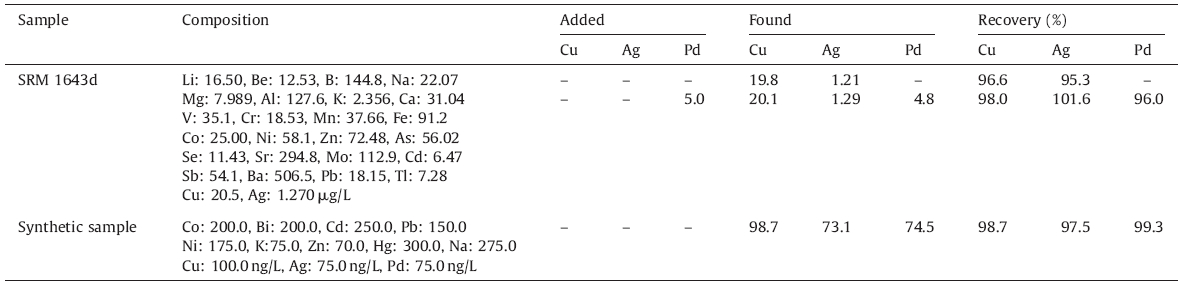
In order to confirm the validity of the developed procedure,this method has also been applied for the determination of the content of Cu,Ag,and Pd in a Standard Reference Material; SRM 1643d.
10 mL of the solution (SRM 1643d) was poured into a 100.0 mL measuring flask,diluted to the mark with deionized water,and the analyte was determined by the proposed method.
Palladium (from its standard stock solution) was added to the SRM 1643d before its preparation. 2.6.3. Synthetic sample
Since no standard samples were accessible for the simultaneous determination of copper,silver,and palladium with the developed method (according to Cu,Ag and Pd linear ranges),the method was applied to a synthetic mixture. Hence,a synthetic mixture containing different cations was prepared. Aliquots of the synthetic sample were taken and the general procedure was applied. 3. Results and discussion
Chelating agents with nitrogen atoms have excellent adsorption properties for heavy metal ions and are applied in the separation,enrichment,and removal of heavy metal ions [8].
Polyethylenimine (PEI) is a polyamine and has a large quantity of nitrogen atoms from amino groups on the line-type and branched structure of PEI,which can produce very strong chelating action. Silver and palladium ions are soft cations; for them,the following order of donor atom affinity is observed: O < N < S. Copper ions are in the borderline cation group that possesses strong affinity for intermediate (N) and soft (S) ligands. Coating alumina with PEI is a cheap and simple method (one step). The results of elemental analysis of the dried adsorbent showed that N and C sites exist,thus alumina was coated with PEI. The experiments showed that the PEI/Al2O3 in neutral media has more adsorption capacity than in acidic or alkaline media. Increasing the ionic strength caused an increase in the amount of PEI at alumina surface at all measured pH values. Therefore, NaNO3 was used to increase the ionic strength. The amount of NaNO3 was optimized,and it was observed that 0.5 g of NaNO3 was suitable for 1.0 g of alumina (Table 2).
3.1. Effect of pH
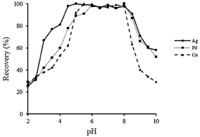
The reaction between metal ions (Cu,Ag,and Pd) and the chelating agent can be influenced by changes in pH. In order to optimize this parameter,the pH of the sample solution was studied in the range of 2.0-11.0,using of HNO3 and KOH for pH adjustment. The results showed that the copper,silver and palladium ions were completely adsorbed on the adsorbent over the pH range of 5.5- 8.0,4.5-8.0 and 6-8.0,respectively (Fig. 1). At very acidic pHs,a majority of the amine sites are protonated and cannot take part in the chelation process,but above pH 8.0,the recoveries are not quantitative because of hydroxide formation of metal ions. So,for simultaneous preconcentration of these three ions,pH 7.0 was chosen as the optimum pH for further studies. Also,for pH adjusting,KH2PO4/K2HPO4 buffer with pH 7.0 was used.
|
Download:
|
| Fig. 1.Effect of pH on the extraction efficiency of Cu, Ag and Pd. Experimental conditions were the same as in Table 3 except the pH. | |
A series of different amounts of the adsorbent (PEI/Al2O3) were used for simultaneous adsorption of 100.0 ng of Cu,Ag,and Pd from 100.0 mL of sample solution. The results showed that for the quantitative extraction of three ions,at least 50.0 mg of the adsorbent is needed. 3.3. Adsorption capacity
The capacity of the adsorbent is an important factor because it determines how much adsorbent is required to quantitatively extract a specific amount of metal ions from the sample solutions. The adsorption capacity of PEI/Al2O3 was determined by passing 100.0 mL of solution containing 1.0 mg of copper,silver,and palladium from 50.0 mg of adsorbent. The amount of Cu,Ag,and Pd ions adsorbed on to the PEI/Al2O3 were calculated by the difference between the initial and the final concentrations of analyte ions in the solution. The maximum adsorption capacity has been found to be 17.0,13.5 and 13.0 mg/g for copper,silver,and palladium, respectively. The difference between the adsorption capacities of the three metal ions can be due to their sizes,degree of hydration, and their charge/radius ratio.
In order to confirm the activity of PEI in the extraction and confirm its deposition onto the surface of Al2O3,the outlined method was tested using unmodified Al2O3. The maximum adsorption capacity of unmodified Al2O3 was found to be 6.6 mg/g for Cu,4.8 mg/g for Ag,and 5.1 mg/g for Pd,which confirms that the PEI was adsorbed on the surface of alumina and the adsorption capacity of PEI-Al2O3 is much higher than unmodified Al2O3. 3.4. Effect of eluent type
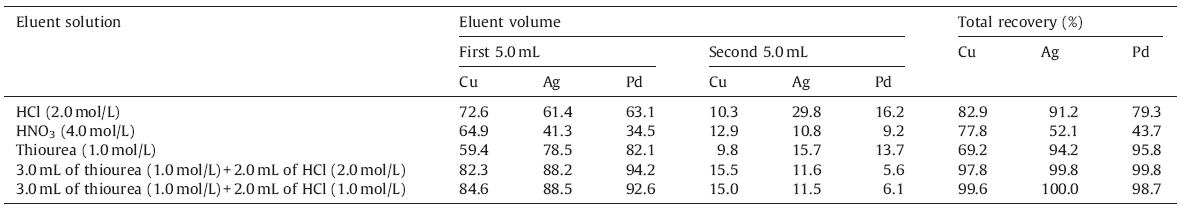
In order to select the most effective eluent for desorption of the retained metal ions,a series of various eluent solutions were studied under the optimum conditions (Table 3). The results showed that quantitative recoveries were obtained using two cycles with 5.0 mL eluent including 3.0 mL of thiourea (1.0 mol/L) followed by one cycle of 2.0 mL of HCl (1.0 mol/L).
| Table 3 The eluent solution type and recovery of copper, silver and palladium ions. |
The sample and eluent flow rates are important parameters to obtain quantitative retention and elution,respectively. The influence of flow rates was investigated in the range of 0.2- 10 mL/min for both the sample and eluent. The retentions for analytes (including 100.0 ng of Cu,Ag,and Pd at pH 7.0) were quantitative up to a sample flow rate of 2.0 mL/min. Also,in the range of 0.2-4.0 mL/min for eluent flow rate,there was no significant difference in the recovery of all three metal ions. Therefore,all further studies were performed at 2.0 mL/min sample flow rate and 4.0 mL/min eluent flow rate. 3.6. Breakthrough volume
When dealing with real samples containing very low concentrations of element ions,in order to obtain high preconcentration factors,the maximum applicable sample volume must be determined. Thus the effect of sample volume on the extraction of 100.0 ng of Cu,Ag,and Pd was studied by taking different samples volumes (500-2500 mL). The extraction was carried out as described earlier. The results show that the recovery of the analytes did not decrease significantly up to 2000,1500,and 2000 mL for Cu,Ag,and Pd,respectively. By using 10.0 mL of eluent,the preconcentration factors of 200,150,and 200 were obtained for Cu,Ag,and Pd,respectively. 3.7. Reusability of modified adsorbent
In order to investigate the effectiveness of the modified adsorbent,PEI/Al2O3 was reused. The experimental results indicate that the recovery of analytes decreases slightly when the adsorbent is reused 8,6,and 9 times for Cu,Ag,and Pd, respectively. 3.8. Effect of interfering ions on recovery
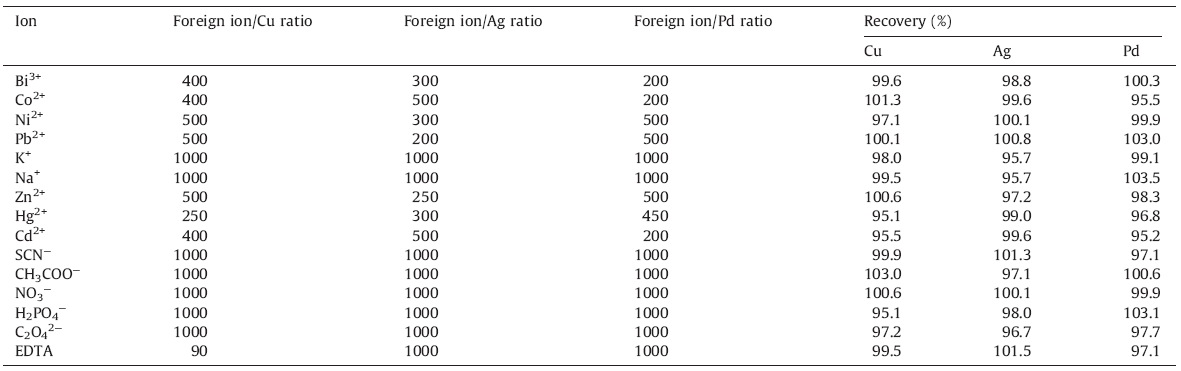
The efficiency of the suggested method in the extraction and preconcentration of the three ions in the presence of various cations and anions was examined by using a solution containing 100.0 ng of Cu,Ag and Pd with addition of various concentrations of potential interferences. The tolerance level was defined as the maximum amount of foreign species producing an error of ±5% in Cu,Ag,and Pd determination. The tolerance level of each potentially interfering ion species was tested,and if interference occurred,the ratio was reduced until it ceased. As can be seen from Table 4,several species did not interfere even at high concentrations,showing that the method described is applicable to the analysis of Cu,Ag,and Pd ions in different samples.
| Table 4 Effect of potentially interfering ions on the extraction efficiency of Cu, Ag and Pd. |
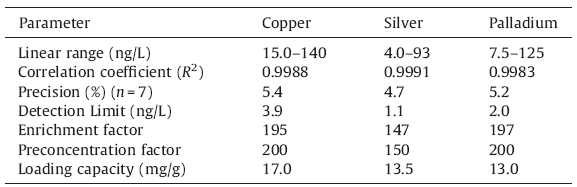
Under the optimum conditions,performance characteristics were obtained by processing standard solutions of copper,silver, and palladium,and the data is illustrated in Table 5.
| Table 5 Analytical characteristics of suggested method. |
To test the reproducibility of the proposed column solid phase extraction method,the suggested procedure was repeated seven times under optimum conditions. The relative standard deviation (R.S.D.) was measured to be ±5.4%,±4.7%,and ±5.2% for Cu,Ag,and Pd,respectively. The calibration curves for the determination of Cu, Ag,and Pd under the optimized conditions show linearity over the range of 15.0-140 ng/L (R2 = 0.9988) for Cu,4.0-93 ng/L (R2 = 0.9991) for Ag,and 7.5-125 ng/L (R2 = 0.9983) for Pd in the original solutions. Also,limit of detections based on 3Sb/m (where Sb is the standard deviation of the blank signals and m is the slope of the calibration curve after extraction) were calculated to be 3.9,1.1,and 2.0 ng/L for Cu,Ag,and Pd,respectively. The enrichment factor,which was calculated based on the slopes of the calibration curves with or without the extraction,was 195 for copper,147 for silver,and 197 for palladium. Also,the preconcentration factors are 200,150,and 200 for Cu,Ag,and Pd,respectively. 3.10. Analytical applications
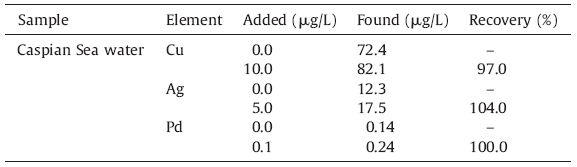
In order to establish the validity of the procedure,the proposed method was applied to the simultaneous extraction of copper, silver,and palladium in Caspian Sea water. The reliability of the method was checked by analysis of the samples spiked with known amounts of Cu,Ag,and Pd in the sea water sample. The results presented in Table 6 reveal that recovery at 95% confidence level is satisfactory.
| Table 6 Analysis of copper, silver and palladium in sea water. |
Also,to verify the accuracy of the method,this procedure was also applied to the determination of Cu,Ag,and Pd in a standard reference material (SRM 1643d) and a synthetic sample. The analytical results are given in Table 7. As can be seen,the obtained results are in good agreement with the reference values,and there is no significant difference between the results and the accepted values. Thus,the procedure is reliable for analysis of a wide range of samples.
| Table 7 Analysis of copper, silver and palladium in synthetic sample and certified reference material. |
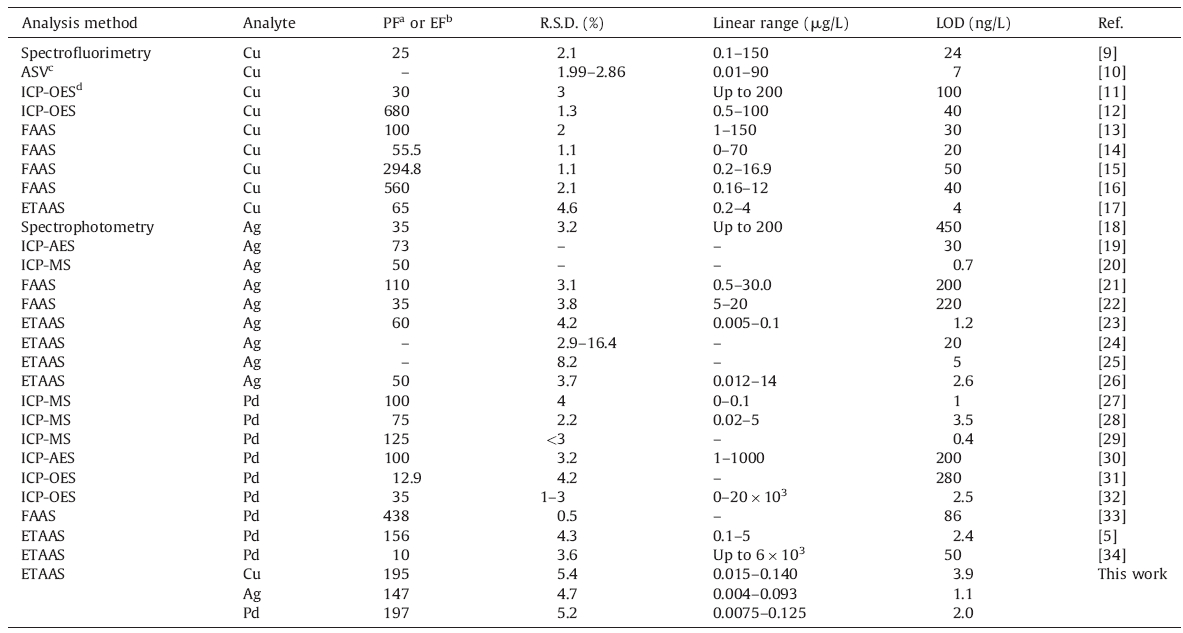
Table 8 compares the characteristic data of the suggested method with other methods for determination of Cu,Ag,and Pd which have been reported in the literature [5, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34]. As can be seen from Table 8,for the three elements,the recommended method possesses a wide linear dynamic range,high sampling volume, high preconcentration factor,and high sensitivity. The proposed method has also the lowest detection limit except the results reported in the following literature [20, 27, 29] and best enrichment factor except the following reported [12, 15, 16, 33],for simultaneous extraction of copper,silver,and palladium ions. Moreover,in all of these reported articles,only one or two elements were determined,whereas the recommended procedure can extract and determine of three metal ions,simultaneously. Finally because of the low toxicity of this method,it is proper to mention this method as a green and environmentally friendly method.
| Table 8 Comparison of the proposed method with other reported methods for preconcentration and determination of copper, silver and palladium. |
In this study,a new adsorbent (PEI/Al2O3) in combination with ETAAS was applied for simultaneous preconcentration and determination of copper,silver,and palladium. It has been shown that these three metal ions can form the complexes with PEI and then,adsorbed on the surface of modified alumina. Besides considerably high preconcentration ability,some other benefits of the system were the simultaneous determination of three elements,enhancement of ETAAS sensitivity,its simplicity and speed of analysis.
| [1] | G. Huand, R.L. Deming, Speciation of bio-available chromium in soils by solidphase extraction and graphite furnace atomic absorption spectrometry, Anal. Chim. Acta 535 (2005) 237-242. |
| [2] | O. Mikkelsen, S.M. Skogvold, K.H.Schrøder, Continouos heavy metal monitoring system for application in river and seawater, Electroanalysis 17 (2005) 431-439. |
| [3] | H. Ashkenani, M.A. Taher, Selective voltammetric determination of Cu(II) based on multiwalled carbon nanotube and nano-porous Cu-ion imprinted polymer, J. Electroanal. Chem.683 (2012) 80-87. |
| [4] | S. Jahandari, M.A. Taher, H. Fazelirad, I. Sheikhshoai, Anodic stripping voltammetry of silver(I) using a carbon paste electrode modified with multi-walled carbon nanotubes, Microchim. Acta 180 (2013) 347-354. |
| [5] | P. Liang, E. Zhao, F.Li, Dispersive liquid-liquid microextraction preconcentration of palladium in water samples and determination by graphite furnace atomic absorption spectrometry, Talanta 77 (2009) 1854-1857. |
| [6] | H. Fazelirad, M.A.Taher, Ligandless, ion pair-based and ultrasound assisted emulsification solidified floating organic drop microextraction for simultaneous preconcentration of ultra-trace amounts of gold and thallium and determination by GFAAS, Talanta 103 (2013) 375-383. |
| [7] | M.A. Cheraghi, H. Taher, Fazelirad, Voltammetric sensing of thallium at a carbon paste electrode modified with a crown ether, Microchim. Acta 180 (2013) 1157-1163. |
| [8] | A.A. Atia, A.M. Donia, A.M. Yousif, Synthesis of amine and thio chelating resins and study of their interaction with zinc(II), cadmium(II) and mercury(II), React, Funct. Polym.56 (2003) 75-82. |
| [9] | M. Zeeb, M.R. Ganjali, P. Norouzi, M.R. Kalaee, Separation and preconcentration system based on microextraction with ionic liquid for determination of copper in water and food samples by stopped-flow injection spectrofluorimetry, Food Chem. Toxicol. 49 (2011) 1086-1091. |
| [10] | S. Abbasi, H. Khani, R. Tabaraki, Determination of ultra trace levels of copper in food samples by a highly sensitive adsorptive stripping voltammetric method, Food Chem. 123 (2010) 507-512. |
| [11] | E.A. Takara, S.D. Pasini-Cabello, S. Cerutti, J.A. Gasquez, L.D. Martinez, On-line preconcentration/determination of copper in parenteral solutions using activated carbon by inductively coupled plasma optical emission spectrometry, J. Pharm. Biomed. Anal.39 (2005) 735-739. |
| [12] | M. Faraji, Y. Yamini, S. Shariati, Application of cotton as a solid phase extraction sorbent for on-line preconcentration of copper in water samples prior to inductively coupled plasma optical emission spectrometry determination, J. Hazard. Mater.166 (2009) 1383-1388. |
| [13] | M.H. Mashhadizadeh, M. Pesteh, M. Talakesh, et al., Solid phase extraction of copper (II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry, Spectrochim. Acta B 63 (2008) 885-888. |
| [14] | B. Buke, U. Divrikli, M. Soylak, L. Elci, On-line preconcentration of copper as its pyrocatechol violet complex on Chromosorb 105 for flame atomic absorption spectrometric determinations, J. Hazard. Mater. 163 (2009) 1298-1302. |
| [15] | M.C. Yebra, N. Carro, A. Moreno-Cid, Optimization of a field flow pre-concentration system by experimental design for the determination of copper in sea water by flow-injection-atomic absorption spectrometry, Spectrochim. Acta B 57 (2002) 85-93. |
| [16] | A.N. Anthemidis, K.G.Ioannou, On-line sequential injection dispersive liquidliquid microextraction system for flame atomic absorption spectrometric determination of copper and lead in water samples, Talanta 79 (2009) 86-91. |
| [17] | K. Alizadeh, S. Zohrevand, A.R. Ghiasvand, et al., Selective homogeneous liquid- liquid extraction and preconcentration of copper(II) into a micro droplet using a benzo-substituted macrocyclic diamide, and its determination by electrothermal atomic absorption spectrometry, Microchim. Acta 168 (2010) 115-121. |
| [18] | X. Wen, L. Kong, M. Chen, et al., A new coupling of spectrophotometric determination with ultrasound-assisted emulsification dispersive liquid-liquid microextraction of trace silver, Spectrochim. Acta A 97 (2012) 782-787. |
| [19] | M. Hosoba, K. Oshita, R.K. Katarina, et al., Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system, Anal. Chim. Acta 639 (2009) 51-56. |
| [20] | R.K. Katarina, T. Takayanagi, M. Oshima, S. Motomizu, Synthesis of a chitosanbased chelating resin and its application to the selective concentration and ultratrace determination of silver in environmental water samples, Anal. Chim. Acta 558 (2006) 246-253. |
| [21] | C.K. Christou, A.N.Anthemidis, Flow injection on-line displacement/solid phase extraction system coupled with flame atomic absorption spectrometry for selective trace silver determination in water samples, Talanta 78 (2009) 144-149. |
| [22] | C.S.T. Arauujo, V.N. Alves, H.C. Rezende, N.M.M. Coelho, Development of a flow system for the determination of low concentrations of silver using Moringa oleifera seeds as biosorbent and flame atomic absorption spectrometry, Microchem. J. 96 (2010) 82-85. |
| [23] | J.L. Manzoori, G.K. Nezhad, Selective cloud point extraction and preconcentration of trace amounts of silver as a dithizone complex prior to flame atomic absorption spectrometric determination, Anal. Chim. Acta 484 (2003) 155-161. |
| [24] | J. Medved, P. Matus, M. Bujdos, J. Kubova, Gold and silver determination in waters by SPHERON Thiol 1000 preconcentration and ETAAS, Chem. Pap. 60 (2006) 27-31. |
| [25] | M.A. Rahmana, S. Kaneco, M.N. Amina, T. Suzuki, K.Ohta, Determination of silver in environmental samples by tungsten wire preconcentration method - electrothermal atomic absorption spectrometry, Talanta 62 (2004) 1047-1050. |
| [26] | H. Ashkenani, M.A. Taher, Use of ionic liquid in simultaneous microextraction procedure for determination of gold and silver by ETAAS, Microchem. J.103 (2012) 185-190. |
| [27] | M. Moldovan, M.M. Gómez, M.A. Palacios, On-line preconcentration of palladium on alumina microcolumns and determination in urban waters by inductively coupled plasma mass spectrometry, Anal. Chim. Acta 478 (2003) 209-217. |
| [28] | J. Fang, L.W. Liu, X.P. Yan, Minimization of mass interferences in quadrupole inductively coupled plasma mass spectrometric (ICP-MS) determination of palladium using a flow injection on-line displacement solid-phase extraction protocol, Spectrochim. Acta B 61 (2006) 864-869. |
| [29] | M.V.B. Krishna, M. Ranjit, K. Chandrasekaran, G. Venkateswarlu, D.Karunasagar, On-line preconcentration and recovery of palladium from waters using polyaniline (PANI) loaded in mini-column and determination by ICP-MS; elimination of spectral interferences, Talanta 79 (2009) 1454-1463. |
| [30] | M.R. Jamali, Y. Assadi, F. Shemirani, M.Salavati-Niasari, Application of thiophene- 2-carbaldehyde-modified mesoporous silica as a new sorbent for separation and preconcentration of palladium prior to inductively coupled plasma atomic emission spectrometric determination , Talanta 71 (2007) 1524-1529. |
| [31] | J. Nakajima, M. Ohno, K. Chikama, T. Seki, K.Oguma, Determination of traces of palladium in stream sediment and auto catalyst by FI-ICP-OES using on-line separation and preconcentration with QuadraSil TA, Talanta 79 (2009) 1050- 1054. |
| [32] | M. Muzikar, C. Fontàs, M. Hidalgo, J. Havel, V.Salvadó, A preconcentration system using polyamine Metalfix-Chelamine resin for the on-line determination of palladium(II) and platinum(IV) by inductively coupled plasma optical emission spectrometry, Talanta 70 (2006) 1081-1086. |
| [33] | H. Ebrahimzadeh, N. Tavassoli, M.M. Amini, Y. Fazaeli, H.Abedi, Determination of very low levels of gold and palladium in wastewater and soil samples by atomic absorption after preconcentration on modified MCM-48 and MCM-41 silica, Talanta 81 (2010) 1183-1188. |
| [34] | B. Godlewska-Zylkiewiczand, M. Zaleska, Preconcentration of palladium in a flow-through electrochemical cell for determination by graphite furnace atomic absorption spectrometry, Anal. Chim. Acta 462 (2002) 305-312. |