1. Introduction
Avermectins (AVMs),as antiparasitic drugs and agricultural pesticides,are a class of neurotoxins macrocyclic lactone compounds [1]. AVMs are widely used in animal and crop protection,such as peanut,corn,cattle and pig,against mites and insects [2]. Accordingly,edible oils originating from plants and animals may contain AVMs residue,causing potential risk towards public health. AVMs are toxic to nervous and growth systems,their harm to the environment and humans has raised more and more concerns on the topic of residue analysis in food and agro-products [3, 4]. A number of standard analytical methods [5, 6] and maximum residue limits (MRLs) [7] have been developed for AVMs monitoring in China. However,there is still no standard detection method or MRL of AVMs in edible oils. Furthermore,to the best of our knowledge the detection of AVMs in edible oils has not been reported. Abamectin (ABA) and ivermectin (IVR) are two most widely used pesticides in AVM group. The lowest MRL of ABA and IVR established by Codex Alimentarius Commission (CAC) was 5 μg/kg and 10 μg/kg,respectively,for milk products while no MRL has been set for edible oils [8, 9]. Therefore,it is necessary and urgent to develop a selective,sensitive and reliable method to determine AVMs in edible oil samples.
Sample preparation is particularly important for the determination of trace analytes in complex samples to reduce interferences from matrices and enhance sensitivity,accuracy and precision of the method [10]. Sample preparation methods reported in AVMs analysis,included solid phase extraction (SPE) [11, 12, 13, 14, 15],QuEChERS [16],solid phase microextraction (SPME) [17, 18],accelerated solvent extraction (ASE) [19, 20],supercritical fluid extraction (SFE) [21],immunoaffinity chromatography [22], as examples. It is well known that fatty components of the matrices cause analytical problems and tend to undermine the method efficiency. The cleanup of lipids usually requires multiple liquid- liquid extractions (LLEs) or a SPE procedure with high consumption of organic solvents and several operation steps. Low temperature purification (LTP) is a simple,economical,relatively eco-friendly sample preparation technique [23, 24, 25],which makes it a potential alternative to traditional methods. The general process of LTP is as follows: after LLE,the co-extracted interference in the extraction phase (organic solvent) is reduced by freezing based on the distinct difference of melting points between sample matrix,for example oil or water,and the extraction solvent. The co-extracted interferences can be separated as precipitated and frozen sample matrix,while analytes are still dissolved in extraction solvent. With the virtues of ease of operation,relatively few manipulation steps and low consumption of solvent,LTP was applied to trace target analysis in complicated samples such as edible oils [26, 27], milk [28, 29],honey [30],tomato [31] bovine muscle [32],human liver [33] and bean sprouts [34]. But thus far,LTP is not yet utilized in the detection of AVMs in edible oils.
In this study,an analytical method based on LTP combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) was established for the determination of ABA and IVR in edible oils. The proposed method is selective,sensitive and accurate with low lost. As far as we know,this is the first paper on the determination of the trace levels of ABA and IVR in edible oils using LTP as the sample preparation method. 2. Experimental 2.1. Instruments and materials
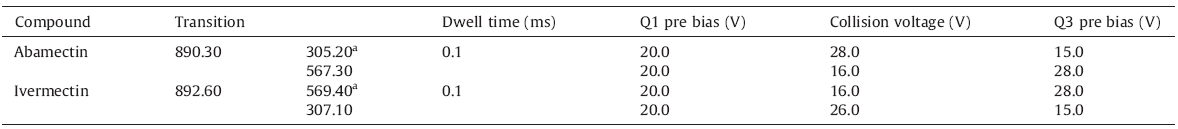
The LCMS-8040 system was a UFLC connected to a triple quadrupole MS analyzer (the second section is a collision chamber) with an electrospray ionization (ESI) interface (Shimadzu,Japan). A Shimadzu Shim-pack XR-ODS (75 mm × 3.0 mm,3.0 μm) column was operated at 40 ℃. Mobile phase was acetonitrile:water (10 mmol/L ammonium formate) = 95:5 (v/v) with a flow rate of 0.4 mL/min. The injection volume was 5 μL. The optimum mass spectrometry operating parameters were as follows: probe voltage,4.5 kV; nebulizing gas,3.0 L/min; drying gas,15 L/min; desolvation temperature,250 ℃; heat block temperature,450 ℃. ABA and IVR were detected in the multiple reaction monitoring (MRM) mode,using the parameters showed in Table 1.
| Table 1 Parameters of MS for the detection of abamectin and ivermectin. |
Chromatographic grade methanol,acetonitrile and n-hexane were from Honeywell International (USA). Ammonium formate, abamectin and ivermectin (purity ≥ 99.0%) were obtained from Sigma-Aldrich Co. LLC. (USA). Alumina-B cartridge (6 mL/ 500 mg) was from Bonna-Agela Technologies Inc. (China). Edible oil samples of different brands were purchased in local retail markets. 2.2. Sample preparation
Liquid-liquid extraction: 2.50 g of oil sample and 2.50 mL of n-hexane were added to a 15 mL centrifuge tube. The mixture was homogenized with a vortex for 1 min,5.00 mL of acetonitrile saturatedwith n-hexane as the extraction solventwas added and the extraction was operated with a vortex for 2 min. Then the sample was centrifuged for 5 min at 4500 rpm before purification.
LTP: The upper organic phase was carefully transferred to another 15 mL centrifuge tube and stored in a refrigerator at -30 ℃ for 16 h to remove fatty interfering components. Finally, 1.00 mL of cold,liquid supernatant was transferred to a bottle and dried with a rotary evaporator. The residue was reconstituted in 1.00 mL acetonitrile and filtered through a nylon filter (0.22 μm) before LC-MS/MS analysis.
SPE purification: An Alumina-B cartridge was conditioned with 5 mL acetonitrile. Then 3.00 mL of extract from LLE procedure was passed through the cartridge. Then the cartridge was washed with 5mL of n-hexane saturated with acetonitrile. The cartridge was eluted twice with 5 mL methanol each time. The eluents were brought to dryness with a rotary evaporator. The residue was recovered in 3.00 mL acetonitrile and filtered through a nylon filter (0.22 μm) before LC-MS/MS analysis. 3. Results and discussion 3.1. Optimization of sample preparation conditions
Acetone,methanol,toluene and acetonitrile could be used to extract ABA and IVR. However,acetone and methanol will dissolve a large amount of lipids which may strongly interfere with chromatographic separation and mass spectrometry detection. Toluene is very toxic,thus acetonitrile is a preferred option as extraction solvent. On the other hand,viscous edible oils were initially dissolved in n-hexane to facilitate the extraction. To optimize the volume of n-hexane,2.50,5.00 and 10.00 mL of nhexane were used to dissolve 2.50 g of oil sample using 5.00 mL of extraction solvent. It turned out that 2.50 mL of n-hexane could dissolve 2.50 g of oil and the sample phase was the bottom layer below the extraction phase after homogenizing,which facilitated the next transfer step. For 5.00 mL of n-hexane,the sample phase and extraction phase could not be separated into two distinct layers. For 10.00 mL of n-hexane,the extraction phase became the lower layer which was not convenient for transfer and might increase interference. Accordingly,2.50 mL of n-hexane was utilized as an optimized parameter.
The entire sample preparation process consumed only 8.50 mL of organic solvent which was a relatively minor amount compared with the reported methods based on LLE-SPE procedures [35, 36] for trace analysis in edible oils. Though LTP samples commonly require hours of time to freeze the sample matrix,a large number of samples can be managed simultaneously requiring only routine device and simple manipulation. 3.2. Calibration curve,limit of detection and limit of quantification
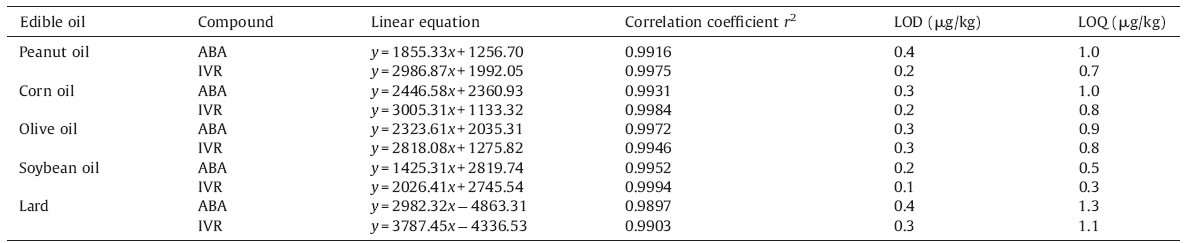
The calibration curves were prepared using matrix-matched standards from 5 μg/L to 1000 μg/L and the results were displayed in Table 2,where y is peak area and x is the concentration (μg/L). The limits of detection (LODs) were three times of the value of signal/noise calculated from the signal/noise value at 5 μg/L,while the limits of quantification (LOQs) were ten times of the value of signal/noise [37, 38]. As could be seen,the LOQs of ABA and IVR in five different matrices were below the lowest MRL established by CAC for milk (5 μg/kg for ABA,10 μg/kg for IVR),while the MRL for oils are not available [8, 9].
| Table 2 Parameters of MS for the detection of abamectin and ivermectin. |
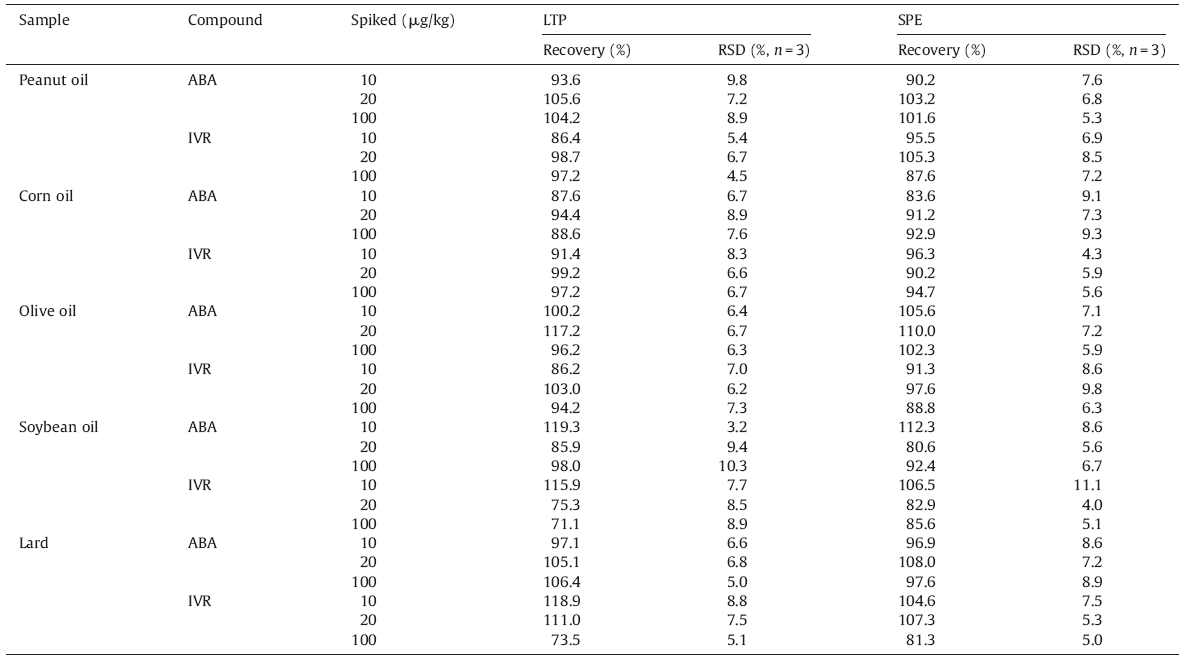
The recoveries of the proposed method were evaluated by analyzing blank samples spiked at three levels of 10,20 and 100 μg/kg in triplicate. As seen in Table 3,the recoveries of ABA were from 85.9% to 119.3% with relative standard deviations (RSDs) of 3.2%-10.3%,while the recoveries of IVR were from 71.1% to 118.9% with RSDs of 4.5%-8.9%. Since there is no published report or standard method on the analysis of avermectins in edible oil,a laboratory method,as mentioned in section 2.2,based on modified SPE purification according to reported works [3, 5, 12] was developed for comparison with the proposed LTP method. The results obtained by LTP method with lower organic solvent consumption and fewer manipulation steps were in agreement with those obtained by SPE method.
| Table 3 Recoveries of abamectin and ivermectin in spiked edible oils. |
The proposed method was applied to the detection of ABA and IVR in peanut oil,corn oil,olive oil,soybean oil and lard. Two samples were examined for each species. Results showed that neither ABA nor IVR was detected in ten real samples and no other peaks interfered with the detection,as shown in the chromatograms in Fig. 1.

|
Download:
|
| Fig. 1.Liquid chromatography–tandem mass spectrometric chromatograms of abamectin and ivermectin in spiked edible oil samples. (a) Blank sample, (b) standard solution of 10 μg/L and (c) sample spiked with 10 μg/kg. | |
The low temperature purification technique is the first reported approach utilized in the analysis of abamectin and ivermectin in edible oils with LC-MS/MS with satisfactory results in terms of sensitivity,selectivity,precision and accuracy. The proposed method proved to be selective,easy to use,requiring few manipulation steps and effective to reduce interference of fatty substances with low consumption of organic solvents and low cost of materials. Therefore,it showed to be appropriate for multiclass analysis of oil samples and a promising alternative to those sample preparation protocols requiring large amount of organic solvent, such as SPE. Its application to other oil matrices and screening for a wider range of pesticides and veterinary drugs should be possible.
AcknowledgmentsThis research was financially supported by National Natural Science Foundation of China (No. 21305019),Special Fund for Agro-Scientific Research in the Public Interest (No. 201303088) and President Fund of Guangdong Academy of Agricultural Sciences (No. 201218).
| [1] | A.I. Valenzuela, M.J. Redondo, Y. Pico, G. Font, Determination of abamectin in citrus fruits by liquid chromatography-electrospray ionization mass spectrometry, J. Chromatogr. A 871 (2000) 57-65. |
| [2] | L. Howells, M.J.Sauer, Multi-residue analysis of avermectins and moxidectin by ion-trap LC-MSn, Analyst 126 (2001) 155-160. |
| [3] | Y. Zhang, C.M. Jiang, H. Chen, et al., Simultaneous detection of abamectin and ivermectin residue in eel with liquid chromatography-tandem mass spectrometry, Chin. J. Anal. Lab. 27 (2008) 104-107. |
| [4] | H.M. He, H. Zhao, C.R. Zhang, et al., Determination of abamectin residues in grain by ultra performance liquid chromatography-tandem mass spectrometry, Chin. J. Anal. Chem. 41 (2013) 1627-1632. |
| [5] | Determination of ivermectin, abamectin, doramectin and eprinomectin residues in fugu, eel and baked eel鈥擫C-MS/MS method, National Standards of the Peoples Republic of China, GB/T 22953-2008. |
| [6] | Determination of ivermectin, abamectin, doramectin and eprinomectin residues in milk and milk powder鈥擫C-MS/MS method, National Standards of the Peoples Republic of China, GB/T 22968-2008. |
| [7] | Maximum residue limits for pesticides in food, National Standards of the Peoples Republic of China, GB 2763-2012. |
| [8] | Report of the Codex Committee on Pesticide Residues, 24th session of the Codex Alimentarius Commission, 2001. |
| [9] | Report of the Codex Committee on Residues of Veterinary Drugs in Foods, 26th session of the Codex Alimentarius Commission, 2003. |
| [10] | G.K. Li, Y.L. Hu, G.H. Ruan, et al., Instruments and Devices of Sample Preparation, Chemical Industry Press, Beijing, 2007. |
| [11] | M.J. Hengel, M. Miller, Analysis of pesticides in dried hops by liquid chromatography- tandem mass spectrometry, J. Agric. Food Chem. 56 (2008) 6851-6856. |
| [12] | J.H. Borges, L.M. Ravelo-Pérez, E.M. Hernández-Suárez, et al., Determination of abamectin residues in avocados by microwave-assisted extraction and HPLC with fluorescence detection, Chromatogrphia 67 (2008) 69-75. |
| [13] | K.A. Krogh, E. Björklund, D. Loeffler, et al., Development of an analytical method to determine avermectins in water, sediments and soils using liquid chromatography- tandem mass spectrometry, J. Chromatogr. A 1211 (2008) 60-69. |
| [14] | H.M. He, H. Zhao, C.R. Zhang, et al., Determination of abamectin residue in paddy rice by ultra performance liquid chromatography-tandem mass spectrometry, Chin. J. Anal. Chem. 40 (2012) 140-144. |
| [15] | J. Hernández-Borges, L.M. Ravelo-Pérez, E.M. Hernández-Suárez, et al., Analysis of abamectin residues in avocados by high-performance liquid chromatography with fluorescence detection, J. Chromatogr. A 1165 (2007) 52-57. |
| [16] | M.L.G. Pérez, R. Romero-González, J.L.M. Vidal, et al., Analysis of veterinary drug residues in cheese by ultra-high-performance LC coupled to triple quadrupole MS/MS, J. Sep. Sci. 36 (2013) 1223-1230. |
| [17] | A. Giordano, M. Fernández-Franzón, M.J. Ruiz, et al., Pesticide residue determination in surface waters by stir bar sorptive extraction and liquid chromatography/ tandem mass spectrometry, Anal. Bioanal. Chem. 393 (2009) 1733-1743. |
| [18] | A.M. Filho, F.N.D. Santos, P.A.D.P. Pereira, Multi-residue analysis of pesticide residues in mangoes using solid-phase microextraction coupled to liquid chromatography and UV-vis detection, J. Sep. Sci. 34 (2011) 2960-2966. |
| [19] | X. Xia, Z.M. Xiao, Q.S.Huang, Simultaneous determination of avermectin and milbemycin residues in bovine tissue by pressurized solvent extraction and LC with fluorescence detection, Chromatographia 72 (2010) 1089-1095. |
| [20] | R.A. Lorenzo, S. Pais, I. Racamonde, et al., Pesticides in seaweed: optimization of pressurized liquid extraction and in-cell clean-up and analysis by liquid chromatography- mass spectrometry, Anal. Bioanal. Chem. 404 (2012) 173-181. |
| [21] | J.H. Park, J.H. Choi, A.M.A. EI-Aty, et al., Development of an extraction method for the determination of avermectins in soil using supercritical CO2 modified with ethanol and liquid chromatography-tandem mass spectrometry, J. Sep. Sci. 36 (2013) 148-155. |
| [22] | X.L. Hou, X.W. Li, S.Y. Ding, et al., Simultaneous analysis of avermectins in bovine tissues by LC-MS-MS with immunoaffinity chromatography cleanup, Chromatographia 63 (2006) 543-550. |
| [23] | C. Lentza-Rizos, E.J. Avramides, F. Cherasco, Low-temperature clean-up method for the determination of organophosphorus insecticides in olive oil, J. Chromatogr. A 912 (2001) 135-142. |
| [24] | S.M. Goulart, M.E.L.R.D. Queiroz, A.A. Neves, J.Dequeiroz, Low-temperature cleanup method for the determination of pyrethroids in milk using gas chromatography with electron capture detection, Talanta 75 (2008) 1320-1323. |
| [25] | C. Lentza-Rizos, E.J. Avramides, E. Visi, Determination of residues of endosulfan and five pyrethroid insecticides in virgin olive oil using gas chromatography with electron-capture detection, J. Chromatogr. A 921 (2001) 297-304. |
| [26] | L. Li, H.Y. Zhang, C.P. Pan, et al., Multiresidue analytical method of pesticides in peanut oil using low-temperature cleanup and dispersive solid phase extraction by GC-MS, J. Sep. Sci. 30 (2007) 2097-2104. |
| [27] | T.D. Nguyen, M.H. Lee, G.H. Lee, Rapid determination of 95 pesticides in soybean oil using liquid-liquid extraction followed by centrifugation, freezing and dispersive solid phase extraction as cleanup steps and gas chromatography with mass spectrometric detection, Microchem. J. 95 (2010) 113-119. |
| [28] | P.D. Andrade, J.L.G.D. Silva, E.D. Caldas, Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and ochratoxin A in breast milk by high-performance liquid chromatography/ fluorescence after liquid-liquid extraction with low temperature purification (LLE-LTP), J. Chromatogr. A 1304 (2013) 61-68. |
| [29] | G. Rübensam, F. Barreto, R.B. Hoff, T.M. Pizzolato, A liquid-liquid extraction procedure followed by a low temperature purification step for the analysis of macrocyclic lactones in milk by liquid chromatography-tandem mass spectrometry and fluorescence detection, Anal. Chim. Acta 705 (2011) 24-29. |
| [30] | G.P.D. Pinho, A.A. Neves, M.E.L.R.D. Queiroz, F.O. Silvoôrio, Optimization of the liquid-liquid extraction method and low temperature purification (LLE-LTP) for pesticide residue analysis in honey samples by gas chromatography, Food Control 21 (2010) 1307-1311. |
| [31] | G.P.D. Pinho, A.A. Neves, M.E.L.R.D. Queiroz, F.O. Silvoôrio, Pesticide determination in tomatoes by solid-liquid extraction with purification at low temperature and gas chromatography, Food Chem. 121 (2010) 251-256. |
| [32] | G. Rübensam, F. Barreto, R.B. Hoff, et al., Determination of avermectin and milbemycin residues in bovine muscle by liquid chromatography-tandem mass spectrometry and fluorescence detection using solvent extraction and low temperature cleanup, Food Control 29 (2013) 55-60. |
| [33] | E.J. Magalhaães, M.E.L.R.D. Queiroz, M.L.D.O. Penido, et al., Determination of cocaine in postmortem human liver exposed to overdose. Application of an innovative and efficient extraction/clean up procedure and gas chromatography- mass spectrometry analysis, J. Chromatogr. A 1309 (2013) 15-21. |
| [34] | S.K. Cho, A.M.A. El-Aty, K.H. Park, et al., Simple multiresidue extraction method for the determination of fungicides and plant growth regulator in bean sprouts using low temperature partitioning and tandem mass spectrometry, Food Chem. 136 (2013) 1414-1420. |
| [35] | F.A. Esteve-Turrillas, A. Pastor, M.D.L. Guardia, Determination of pyrethroid insecticide residues in vegetable oils by using combined solid-phases extraction and tandem mass spectrometry detection, Anal. Chim. Acta 553 (2005) 50-57. |
| [36] | E.G. Amvrazi, T.A. Albanis, Multiresidue method for determination of 35 pesticides in virgin olive oil by using liquid-liquid extraction techniques coupled with solid-phase extraction clean up and gas chromatography with nitrogen phosphorus detection and electron capture detection, J. Agric. Food Chem. 54 (2006) 9642-9651. |
| [37] | S. Jiang, Y.S. Li, B. Sun, Determination of trace level of perchlorate in Antarctic snow and ice by ion chromatography coupled with tandem mass spectrometry using an automated sample on-line preconcentration method, Chin. Chem. Lett. 24 (2013) 311-314. |
| [38] | L.N. Chen, F.R. Song, Z. Zheng, et al., Studies on the determination method of pesticide multi-residues in ginseng by ultra performance liquid chromatography tandem mass spectrometry, Acta Chim. Sin. 70 (2012) 843-851. |