b University of Chinese Academy of Sciences, Beijing 100049, China
1. Introduction
The activation energy is the minimum amount of energy required to initiate a reaction. It is one of the important indexes for appraising a reaction [1]. The chemical reaction rate is related closely to the value of activation energy. The lower the activation energy,the faster the reaction rate is. Reducing activation energy is propitious to promoting a chemical reaction. Ultrasound irradiation [2, 3, 4, 5, 6, 7, 8] and catalyst [9] are two common ways to reduce activation energy.
As a common anisotropic wet etching process applied widely in the semiconductor industry,making use of the anisotropy of silicon in KOH solution could fabricate lots of micro-nanostructure such as V-groove [10],grating with small blaze angle [11, 12, 13] and echelon grating [14, 15, 16]. As an important intrinsic parameter,quantitative analysis of activation energy could identify the changing law of the etching rate and the effect of temperature on the etching rate.Much significant work has been done to study the relationship between the activation energy and the reaction temperature and concentration. Seidel [17] found that the activation energy of (1 0 0) crystal plane and (1 1 0) crystal plane is 0.595 eV and 0.60 eV,respectively, in his study about the etching reaction of silicon in KOH solution. Herr [18] found that the activation energy of (2 1 1) crystal plane is 0.641 eVwhenthemolar concentrationof theKOHsolutionis6 mol/L and the range of reaction temperature is 50-90 ℃. Zavrocky [19] found that the activation energy of (1 0 0) crystal plane is 0.56 eV when the range of mass fraction of the KOH solution is 25-45%. Furthermore,Price [20] has also studied the quantitative relationship between the activation energy in a Si-KOHreaction systemand IPA. The experimental results indicated that the activation energy of (1 0 0),(1 1 0) and (1 1 1) crystal plane is 0.77 eV,0.76 eV and 0.59 eV,respectively,when the mass fraction of the KOH solution and IPA is 23.4% and 13.3%,respectively.
In the process of silicon wet etching,the ultrasonic vibration is usually introduced to produce low roughness surface and reduce the ‘‘pseudo-mask’’ problem caused by hydrogen bubbles. Several authors such as Ohwada [21, 22, 23, 24] found that the ultrasonic vibration could increase the etching rate during the silicon wet etching process. However,the relationship between the activation energy and the ultrasound frequency and power has not yet been reported in the literature. Furthermore,Jiao [25] indicated that activation energy changed byultrasoundis anessential parameter inthemodel discussing the ultrasonic enhancement on the mass transfer in the silicon-KOH reaction,which means that it is important to confirm the activation energy in a system induced by ultrasound.
In the present work,the effects of ultrasound frequency and power on activation energy in the reaction between Si (1 0 0),Si (1 1 1) and KOH solution have been discussed for the first time. Under the experimental conditions of the confirmed ultrasound frequency and power,we have measured the etching depth of silicon wafer using AFM and solved the etching rate by calculating the ratio of etching depth to etching time. After calculating the activation energy according to the Arrhenius equation,the relation between activation energy and ultrasound frequency and power have been discussed.
2. Experimental
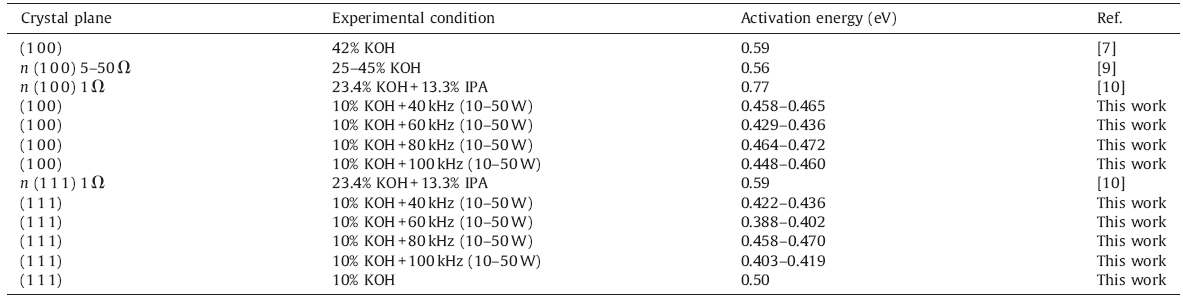
The silicon wafer used in our experiment is high-purity float zone silicon boules up to 76.2 mm in diameter and 1 mm in thickness with a resistivity of approximately 2000 V cm,and a SiO2 layer with a thickness of 70 nm is deposed on silicon wafer using the thermal growth method. The SiO2 mask is formed on the silicon substrate by exposure and development of photoresist and etching on SiO2 layer using the BHF and RIE methods [26]. The samples were etched in an ultrasonic bath. The temperature was varied between 20 ℃ and 60 ℃ and was controlled with an accuracy of ± 0.2 ℃. The mass fraction of KOH solution is 10%. In order to keep the solution concentration uniform,the condenser pipe is added during the wet etching process. The range of ultrasound frequency and ultrasound power is 40-100 kHz and 10-50 W, respectively. Fig. 1 is a sketch showing the experimental equipment in the silicon wet etching process.
|
Download:
|
| Fig. 1.The sketch showing experimental equipment in silicon wet etching process. | |
The silicon wafers were etched under different experimental conditions through changing ultrasound frequency and ultrasound power,and the etching time is 3 min. We have measured the etching depth of silicon wafer using AFM and solved the etching rate by calculating the ratio of etching depth to etching time. Finally we have calculated the activation energy using the least square method in the form of the Arrhenius equation [25, 27].
3. Results and discussion
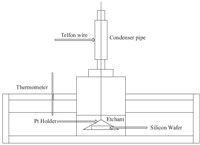
Fig. 2a shows the temperature dependence of the etching rate of (1 0 0) orientated single-crystal silicon in the KOH solution with frequencies at 40 kHz,60 kHz,80 kHz and 100 kHz (power is 50 W) and the same data plotted in Arrhenius form are shown in Fig. 2b. According to the data shown in Fig. 2a,the etching rate R is strongly dependent on temperature T. In this case,the etching rate R is thermally activated,with a relationship to temperature T as given in the equation R = R0 exp(-Ea/kT),where R0 is the frequency factor,Ea is the activation energy,and k is the Boltzmann’s constant. Based upon the experimental data shown in Fig. 2b,the activation energy of silicon wet etching with KOH solution under the conditions of ultrasonic frequencies at 40 kHz,60 kHz,80 kHz and 100 kHz (power is 50 W) was 0.458 eV,0.429 eV,0.464 eV and 0.448 eV,respectively. Fig. 2c,d is the temperature dependence of the etching rate of (1 1 1) orientated single-crystal silicon in the KOH solution with frequencies at 40 kHz,60 kHz,80 kHz and 100 kHz (power is 50 W) and the same data plotted in Arrhenius form respectively. As shown,the activation energy is 0.422 eV, 0.388 eV,0.458 eV and 0.403 eV,respectively. The standard deviation of the activation energy of silicon (1 0 0) is smaller than 1 × 10-11 (eV) and that of silicon (1 1 1) is smaller than 5 × 10-13(eV).
|
Download:
|
| Fig. 2.(a) Temperature dependence of the etching rate along (1 0 0) orientation with different ultrasound frequency; (b) the same data plotted in Arrhenius form; (c) temperature dependence of the etching rate along (1 1 1) orientation with different ultrasound frequency; (d) the same plotted in Arrhenius form (ultrasound power is 50 W). | |
Hydrogen bubbles generated during the reaction between silicon and KOH solution without ultrasound would log on the etched surface to form the ‘pseudo-mask’,which will obstruct the chemical reactions between them and lower the etching rate. The vibration of hydrogen bubbles by ultrasonic cavitation could increase the leaving rate of hydrogen bubbles from silicon surface, and furthermore increase the reaction rate between silicon and KOH solution. Comparing the activation energy we calculated in our paper with the traditional ones,the introduction of ultrasound in the reaction between silicon and KOH solution could reduce the activation energy and increase the reaction rate,which is consistent with above-mentioned results discussed qualitatively. Although the enhancement effects of ultrasound increase with the increase of ultrasound frequency,the experimental results in our work indicated that this enhancement was nonlinear. In the frequency range studied,the enhancement effects of ultrasound on activation energy decrease in the order of 60 kHz,100 kHz,40 kHz and 80 kHz.
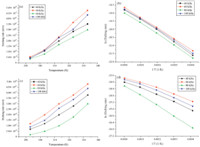
Fig. 3a,b shows the relationship between the activation energy and the ultrasound power under different ultrasound frequencies with Si (100) and Si (1 1 1),respectively. As seen,the activation energy decreases with the increase of ultrasound power at the same ultrasound frequency.

|
Download:
|
| Fig. 3.The sketch showing relationship between activation energy and ultrasound power with (a) Si (1 0 0) and (b) Si (1 1 1). | |
Table 1 shows the activation energy of reaction between Si (1 0 0),Si (1 1 1) and KOH solution under various experimental conditions. To the Si (1 0 0) crystal surface,the IPA added in KOH solution has the effect of increasing activation energy and decreasing the etching rate,and the ultrasonic vibration introduced in the reaction process could reduce the activation energy, increase the etching rate and shorten the etching time.
| Table 1 The activation energy of reaction between Si (1 0 0),Si (1 1 1) and KOH solution under various experimental conditions. |
To the best of our knowledge,the activation energy of the Si (1 1 1) crystal surface without ultrasonic vibration has not been reported before. Based upon this actuality,we calculated the activation energy of Si (1 1 1) crystal surface without ultrasonic vibration in our work and the results are shown in Table 1. Comparing the activation energy of Si (1 1 1) crystal surface with and without ultrasound revealed a similar trend to the Si (1 0 0) crystal surface,that is,the ultrasonic vibration introduced in the reaction process could reduce the activation energy,increase the etching rate and shorten the etching time.
4. Conclusion
The relationship between activation energy of Si (1 0 0),Si (1 1 1)-KOH reaction system and ultrasound frequency and power was discussed for the first time in our work. The experimental results indicated that the enhancement effects of ultrasound on activation energy decrease in the order of 60 kHz,100 kHz,40 kHz and 80 kHz,and the activation energy decreases with the increase of ultrasound power at the same ultrasound frequency.
Acknowledgments
This work is supported by the Project Developing Key Scientific Instrument and Equipment of China (No. 2011YQ120023).
| [1] | C.S. Zhou, H. Ma, Ultrasonic degradation of polysaccharide from a red algae (Porphyra yezoensis), J. Agric. Food Chem. 54 (2006) 2223-2228. |
| [2] | H.M. Moghaddam, S. Nasirian, Decreasing of the activation energy of TiO2 nanoparticles by applying ultrasound waves using the sol-gel method, Iran. J. Phys. Res. 11 (2012) 411-416. |
| [3] | S.U. Rege, R.T. Yang, C.A. Cain, Desorption by ultrasound: phenol on activated carbon and polymeric resin, AIChE J. 44 (1998) 1519-1528. |
| [4] | E.X. Leaes, D. Lima, L. Miklasevicius, et al., Effect of ultrasound-assisted irradiation on the activities of a-amylase and amyloglucosidase, Biocatal. Agric. Biotechnol. 2 (2013) 21-25. |
| [5] | M. Souza, E.T. Mezadri, E. Zimmerman, et al., Evaluation of activity of a commercial amylase under ultrasound-assisted, Ultrason. Sonochem. 20 (2013) 89-94. |
| [6] | M.A. Behnajady, N. Modirshahla, M. Shokri, B. Vahid, Investigation of the effect of ultrasonic waves on the enhancement of efficiency of direct photolysis and photooxidation processes on the removal of a model contaminant from textile industry, Global NEST J. 10 (2008) 8-15. |
| [7] | M.R. Wang, L. Jiang, S.F. Zhou, Z.Y. Zhang, Z.C. Ji, Ultrasound-assisted synthesis and preliminary bioactivity of novel 2H-1,2,4-thiadiazolo [2,3] pyrimidine derivatives containing fluorine, Chin. Chem. Lett. 23 (2012) 561-564. |
| [8] | M.R.P. Heravi, An efficient fluorination of b-ketosulfones promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation using SelectfluorTM F-TEDA-BF4, Chin. Chem. Lett. 21 (2010) 1399-1402. |
| [9] | C.L. Pieck, R.J. Verderone, E.L. Jablonski, J.M. Parena, Burning of coke on Pt Re/Al2O3 catalyst: activation energy and oxygen reaction order, Appl. Catal. 55 (1989) 1-10. |
| [10] | W.T. Tsang, S. Wang, Preferentially etched diffraction gratings in silicon, J. Appl. Phys. 46 (1975) 2163-2166. |
| [11] | J. Sarathy, D.C. Diaz, J.C. Campbell, Crystallographically limited submicrometer gratings in (1 0 0) and (2 1 1) silicon, Opt. Lett. 20 (1995) 1216-1218. |
| [12] | C.H. Chang, R.K. Heilmann, R.C. Fleming, et al., Fabrication of sawtooth diffraction gratings using nanoimprint lithography, J. Vac. Sci. Technol. 21 (2003) 2755- 2759. |
| [13] | M.P. Kowalski, R.K. Heilmann, M.L. Schattenburg, et al., Near-normal-incidence extreme-ultraviolet efficiency of a flat crystalline anisotropically etched blazed grating, Appl. Opt. 45 (2006) 1676-1679. |
| [14] | U.U. Graf, D.T. Jaffe, E.J. Kim, et al., Fabrication and evaluation of an etched infrared diffraction grating, Appl. Opt. 33 (1994) 96-102. |
| [15] | L.D. Keller, D.T. Jaffe, O.A. Ershov, B. Thomas, U.U. Graf, Fabrication and testing of chemically micromachined silicon echelle gratings, Appl. Opt. 39 (2000) 1094- 1105. |
| [16] | J.P. Marsh, D.J. Mar, D.T. Jaffe, Production and evaluation of silicon immersion gratings for infrared astronomy, Appl. Opt. 46 (2007) 3400-3416. |
| [17] | H. Seidel, L. Csepregi, A. Heuberger, H. Baumgartel, Anisotropic etching of crystalline silicon in alkaline solutions. I. Orientation dependence and behavior of passivation layers, J. Electrochem. Soc. 137 (1990) 3612-3626. |
| [18] | E. Herr, H. Baltes, KOH etching of high-index crystal planes in silicon, Sens. Actuators A: Phys. 31 (1992) 283-287. |
| [19] | P.M. Zavrocky, T. Earles, N.L. Pokrovskiy, J.A. Green, B.E. Burns, Fabrication of vertical sidewalls by anisotropic etching of silicon (1 0 0) wafers, J. Electrochem. Soc. 141 (1994) 3182-3188. |
| [20] | J.B. Price, Anisotropic etching of silicon with KOH-H2O-isopropyl alcohol, in: H.R. Huff, R.R. Burgess (Eds.), Semiconductor Silicon, Electrochemical Society, Pennington, 1973, p. 339. |
| [21] | K. Ohwada, Y. Negoro, Y. Konaka, T. Oguchi, Groove depth uniformization in[1 1 0] Si anisotropic etching by ultrasonic wave and application to accelerometer fabrication, in: Proceedings of the IEEE Micro Electro Mechanical Systems, Amsterdam, Netherlands, IEEE, 1995, pp. 100-105. |
| [22] | T. Baum, D.J. Schiffrin, AFM study of surface finish improvement by ultrasound in the anisotropic etching of Si(1 0 0) in KOH for micromachining application, J. Micromech. Microeng. 7 (1997) 338-342. |
| [23] | J. Chen, L.T. Lin, Z.J. Li, et al., Study of anisotropic etching of (1 0 0) Si with ultrasonic agitation, Sens. Actuators A 96 (2002) 152-156. |
| [24] | C.R. Yang, P.Y. Chen, Y.C. Chion, R.T. Lee, Effects of mechanical agitation and surfactant additive on silicon anisotropic etching in alkaline KOH solution, Sens. Actuators A 119 (2005) 263-270. |
| [25] | Q.B. Jiao, Bayanheshig, X. Tan, J.W. Zhu, Numerical simulation of ultrasonic enhancement on mass transfer in liquid-solid reaction by a new computational model, Ultrason. Sonochem. 21 (2014) 535-541. |
| [26] | R.L. Bristol, J.A. Britten, R. Hemphill, P. Jelinsky, M. Hurwitz, Silicon diffraction gratings for use in the far and extreme-ultraviolet, Proc. SPIE 3114 (1997) 580- 585. |
| [27] | J. Peng, C. Chao, J.Y. Dai, H.L.W. Chan, H.S. Luo, Micro-patterning of 0.70Pb (Mg1/3Nb2/3)O3-0.30PbTiO3 single crystals by ultrasonic wet chemical etching, Mater. Lett. 62 (2008) 3127-3130.omers for use in thin film transistors, Chem. Rev. 107 (2007) 1066-1096. |