b Department of Polymer Science & Materials, Dalian University of Technology, Dalian 116012, China
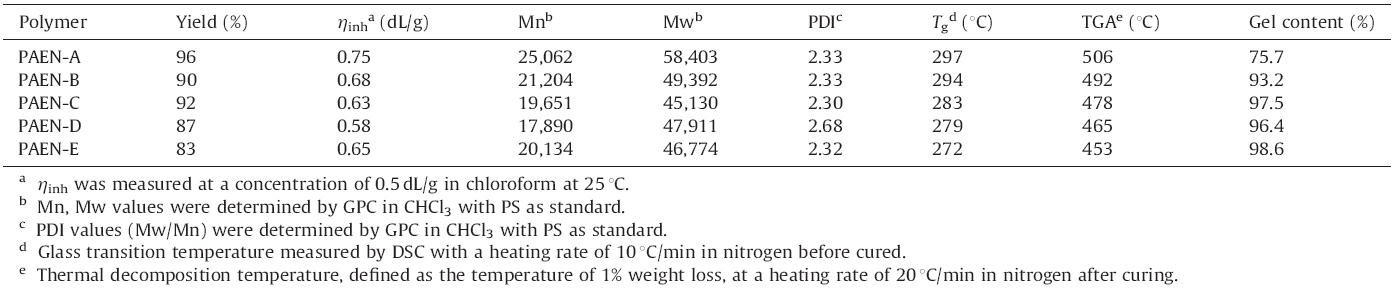
Poly(aryl ether nitrile)s (PAENs) are a class of high performance polymers that have attracted much attention as structural materials in aerospace and electronic industries because of their outstanding mechanical properties,high thermal stability,good chemical inertia and excellent radiation resistance [1, 2]. In addition,the cyano groups may also function as potential crosslinking sites,provide potential site for further functionalizations, and promote adhesion of the polymers to many substrates via interactions with other polar chemical groups [3, 4]. On the one hand,a large number of PAENs have extremely high glass transition temperature and poor solubility in organic solvents, thus making them difficult to be used as thin membranes and coatings. In our previous study,we synthesized a series of PAENs containing 4-(4-hydroxylphenyl)(2H)-phthalazin-1-one (DHPZ) moiety,which behaves like a biphenol to give good solubility and high rigidity to the polymer backbone [5]. On the other hand, the products based on these soluble polymers suffer from poor solvent-resistance. The introduction of crosslinkable moieties into the polymer chains is often adopted as an efficient approach to improve the solvent-resistance,dimensional stability and enhance the Tg values [6]. Allyl bisphenols,such as 2,20-bis(4-allyloxy phenyl)propane (APP) [7],4,40-allyloxybiphenyl (ABP) [8, 9],are important crosslinkable moieties for poly(arylene ether)s and can be synthesized by the aromatic nucleophilic substitution (SNAr) reactions. In this work,a new phthalazinone monomer containing an allyl group (4-(3-allyl-4-hydroxyphenyl)phthalazin-1(2H)-one) (allyl-DHPZ) was synthesized. The PAENs with different crosslinkable group content were synthesized by copolycondensation of bisphenol monomer with 2,6-diflurobenzonitrile (DFBN). Both the synthesis and thermal crosslinking behavior of this crosslinkable polymer were discussed. 2. Experimental 2.1. Allyl-DHPZ synthesis
DHPZ (Dalian Polymer New Materials Co.,Ltd.),NaOH and deionized water were constantly stirred and heated to 70℃ until it is nearly dissolved. Then the allylbromide was added to the reaction mixture. After 8 h,cold water was added and the precipitated powders were collected,washed for several times with boiling 95% ethanol,finally filtered,and dried in an oven to give 4-(4-(allyloxy)phenyl)phthalazin-1(2H)-one (mp 168-169℃, yield 91.3%). FTIR (KBr,cm-1): ν 3021 (s,=CH),2931 (s,-CH2), 2233 (C≡N),1689 (C=O),1261 (m,C-O-C); 1H NMR (400 MHz, CDCl3): δ 4.63 (d,2H),5.30-5.53 (m,2H),6.07-6.17 (m,1H),6.99- 7.13 (m,2H),7.49-7.61 (m,2H),7.72-7.88 (d,3H),8.56 (d,1H), 10.73 (d,1H); 13C NMR (100 MHz,CDCl3): δ 77.14,114.82 (2C), 117.89,126.72,127.16,127.66,128.29,129.31,130.76,131.26, 132.51,132.72,132.97,158.84,159.33.
4-(4-(Allyloxy)phenyl)phthalazin-1(2H)-one obtained in the previous step was kept at 200℃ under N2 for 8 h and the mixture was cooled to room temperature. The precipitates were collected and purified by crystallization from boiling DMAc. The product was obtained as a white powder. Yield 81.3%; MS: 278.1066; FTIR (KBr, cm-1): ν 3200-3600 (O-H),3020 (s,=CH),2930 (s,-CH2),2235(C≡N),1688 (C=O); 1H NMR (400 MHz,CDCl3): δ 3.35 (d,2H), 4.96-5.14 (m,2H),5.91-6.06 (m,1H),6.99 (m,1H),7.23-7.34 (m, 2H),7. 73 (d,1H),7.86-7.94 (m,2H),8.33 (d,1H),9.76 (d,1H), 12.71 (d,1H); 13C NMR (100 MHz,CDCl3): δ 41.71,114.62,115.67, 125.77,126.02,126.26,126.69,127.94,128.31,129.22,130.64, 131.35,133.53,136.83,158.72,159.17. 2.2. Polymer synthesis
The PAEN (A-E) with different crosslinkable group content were synthesized by SNAr polycondensation of DFBN with various content of allyl-DHPZ and DHPZ (Scheme 1). The proportions of the allyl-DHPZ/DHPZ were 0.05/0.95,0.1/0.9,0.2/0.8,0.3/0.7,0.4/0.6, respectively. PAENs with inherent viscosities ranging from 0.58 dL/g to 0.75 dL/gwere readily obtained. GPC analysis of the resulting polymers revealed that they had an averaged molecular weight of over 17,890 and polydispersities in the range of 2.3-2.7 (Table 1).
|
Download:
|
| Scheme 1.Synthetic routes of PAENs. Reagents and conditions: (a) NaOH/H2O,70℃,8 h; (b) N2,200℃,8 h; (c) K2CO3/DMSO,150℃. | |
| Table 1 Properties of PAEN. |
The crosslinkable PAENs were firstly dissolved in chloroform at a concentration of 30 wt%. Then the films were casted from the solution and dried at room temperature under vacuum. The thermal cross-linking processes of the films were then performed by heating the polymer films at 280℃ for 2 h under vacuum. 3. Results and discussion
Traditionally,the O,O0-diallyl phenols are formed by the thermal Claisen rearrangement of the corresponding bisallyloxy ethers [10]. The N-H groups (2) in the DHPZ proved to behave like phenolic O-H groups (1) [11]. Similarly,the monomer DHPZ with two allyl groups should react,like bisphenols via a novel N-allyl coupling reaction,with allylbromide using anhydrous K2CO3 as a base in dimethyl sulfoxide (DMSO). Due to the weaker nucleophilicity of the N- in phthalazinone,we were not able to allylate this nitrogen. In this work,4-(4-(allyloxy)phenyl)phthalazin-1(2H)- one was synthesized using NaOH as a base in water. In the 1H NMR spectrum,the chemical shifts and assignments were consistent with the proposed structure of the monomer (Fig. 1).

|
Download:
|
| Fig. 1.The 1H NMR spectra of monomers and polymer. | |
The polycondensations used typical SNAr procedures in the presence of DMSO and K2CO3 as the solvent and base,respectively. The allyl group isomerized into a 1-propenyl group under these conditions,which is a known transformation. The crosslinking density could be readily controlled by adjusting the ratio of allyl- DHPZ to DHPZ.
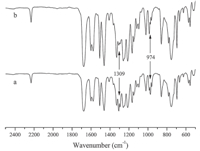
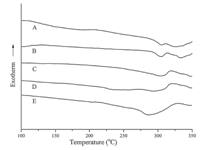
The solubility tests of both powdered virgin and cured PAENs were also conducted to investigate the crosslinking behavior. The virgin PAEN A-E exhibited good solubility in polar organic solvents such as CHCl3,DMAc,DMF,NMP at room temperature. Therefore,it is straightforward to prepare films,coatings or fibers from the solutions of uncross-linked polymers. Both the cured powders and cured films become insoluble in organic solvents. Testing gel content of cured polymers can be done using a direct method to evaluate the content of curing reaction. Thus the gel content of the powdered cured samples was tested by Soxhlet extraction according to the ASTM D2765 method. As shown in Table 1,the gel contents increased with the increase of the crosslinkable propenyl group content. In Fig. 2,The characteristic absorption peaks of the propenyl group near 1309 cm-1 and 974 cm-1 caused by the out of plane vibrations of the "-CH=CH-" almost disappeared totally after curing,indicating that propenyl pendants are efficient crosslinking groups for the synthesized polymers. The glass transition temperature (Tg) of virgin PAEN A-E was measured by DSC at a heating rate of 10℃/min under nitrogen atmosphere,and the DSC scanning curves are shown in Fig. 3. Their DSC heating scans exhibited only one distinct Tg in the range of 272-297℃. A similar trend of decrease in Tg with the increase of the allyl-DHPZ content was observed,indicating that the propenyl group can defect the chair sequence. When temperature exceeds Tg,there are one obvious exothermal peak caused by the crosslinking reaction of propenyl pendants. This means that virgin PAENs barely undergo thermal curing until the moving ability of the rigid backbone increased at higher temperatures. Additionally, the exothermic peaks observed in the first scan disappear in the second DSC scan. These results also clearly indicate the occurrence of certain thermally activated crosslinking of the copolymers. The thermal stability of the cured copolymers was investigated using TGA at a heating rate of 20℃/min under nitrogen atmosphere. Their onset temperatures of 1% weight loss are above 450℃ (Table 1),indicating good thermal stability of the copolymers.
|
Download:
|
| Fig. 2.FTIR spectra of PAEN-E (a) before curing (b) after curing at 280℃ for 2 h under vacuum. | |
|
Download:
|
| Fig. 3. The DSC scanning curves of the PAEN A-E. | |
A new monomer with an allyl group,i.e. 4-(3-allyl-4-hydroxyphenyl) phthalazin-1(2H)-one,was synthesized via a Claisen rearrangement reaction from 4-(4-(allyloxy)phenyl)phthalazin- 1(2H)-one,which was prepared by a nucleophilic displacement reaction with allyl bromide in aqueous NaOH. A novel class of crosslinkable poly(aryl ether nitrile)s with relatively high molecular- weights and good solubility were successfully synthesized by copolymerization of DHPZ,allyl-DHPZ with DFBN. The thermal curing of the synthesized polymers was investigated in situ using differential scanning calorimetry and solubility. The experimental results showed that propenyl pendants are efficient crosslinking groups for the synthesized polymers. The crosslinked polymer exhibits obvious improvements in solvent-resistance and thermal properties. The synthesized high temperature polymer can be used as the base material for high temperature adhesive,coating, composites,proton exchange membranes and the optical matrixes for constructing waveguide devices. Acknowledgments
The project was supported by the Natural Science Foundation of Jiangsu Province (No. BK2012142) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 11KJD150005).
| [1] | V. Lakshmana Rao, A. Saxena, K.N. Ninan, Poly (arylene ether nitriles), J. Macromol. Sci. C: Polym. Rev. 42 (2002) 513-540. |
| [2] | S. Matsuo, T. Murakami, R. Takasawa, Synthesis and properties of new crystalline poly (arylene ether nitriles), J. Polym. Sci. A: Polym. Chem. 31 (1993) 3439-3446. |
| [3] | H.L. Tang, J. Yang, J.C. Zhong, R. Zhao, X.D. Liu, Synthesis and dielectric properties of polyarylene ether nitriles with high thermal stability and high mechanical strength, Mater. Lett. 65 (2011) 2758-2761. |
| [4] | H.L. Tang, X. Huang, X.L. Yang, et al., An effective approach to enhance temperature independence of dielectric properties for polyarylene ether nitrile films, Mater. Lett. 75 (2012) 218-220. |
| [5] | M.J. Wang, C. Liu, L.M. Dong, X.D. Gao, Synthesis and characterization of new soluble poly(aryl ether nitrile)s containing phthalazinone moiety, Chin. Chem. Lett. 18 (2007) 595-597. |
| [6] | Y.W. Yu, X. Yan, M.M. Guo, B.J. Liu, Z.H. Jiang, Allyl-containing poly(aryl ether sulfone): synthesis and crosslinking, Chem. Res. Chin. Univ. 27 (2011) 520-523. |
| [7] | S.L. Zhong, C.G. Liu, H. Na, Preparation and properties of UV irradiation-induced crosslinked sulfonated poly(ether ether ketone) proton exchange membranes, J. Membr. Sci. 326 (2009) 400-407. |
| [8] | G.H. Li, J.Y. Wang, G.P. Yu, et al., Synthesis and characterization of partly fluorinated poly(phthalazinone ether)s crosslinked by allyl group for passive optical waveguides, Polymer 51 (2010) 1524-1529. |
| [9] | F.C. Ding, S.J. Wang, M. Xiao, D.M. Han, Y.Z. Meng, Synthesis and characterization of cross-linkable poly(phthalazinone ether ketone)s, J. Appl. Polym. Sci. 106 (2007) 1821-1827. |
| [10] | C.P. Reghunadhan Nair, K. Krishnan, K.N. Ninan, Differential scanning calorimetric study on the Claisen rearrangement and thermal polymerisation of diallyl ether of bisphenols, Thermochim. Acta 359 (2000) 61-67. |
| [11] | Y.Z. Meng, A.R. Hlil, A.S. Hay, Synthesis and thermal properties of poly(arylene ether ketone)s containing phthalazinone moieties, J. Polym. Sci. A: Polym. Chem. 37 (1999) 1781-1788. |