Isotactic polypropylene (iPP) is one of the most in-demand polymeric materials due to its excellent physical properties and high performances in commercial applications. However,some inherent shortages of iPP,such as poor adhesion,incompatibility, low surface energy and difficulty for chemical modification due to semicrystalline morphology and the lack of chemical functionality, obstruct its commercial success inother fields [1, 2, 3]. Therefore one hot topic in present research is to prepare polar functional polypropylene or polypropylene copolymers without disturbing the isotactic chain structure [4]. To improve the compatibility,one possible way isto synthesize the block orgraft copolymers,but itis a challenging task for synthetic chemistry due to iPP’s special property [5].
It is a very beneficial task to prepare iPP containing terminal functional groups [6, 7] because it can guarantee isotactic chain structure unperturbed,and consequently maintains the functio- nalized iPP with high crystallinity. Moreover,the terminal functional group,having good mobility and reactivity,can endow iPP with desirable interactive properties,forming graft and block copolymers,which can be widely used in many fields [8, 9, 10, 11]. Therefore,various functional groups have been introduced to the end of polypropylene chain [12, 13, 14, 15, 16, 17],such as amino,vinyl,halogen and metal (Al,Zn). However,there are few methods to prepare blockcopolymersoriginatingfromterminal-functionalizediPPdue to the large steric hindrance and heterogeneous reaction condi- tions.
Free-radical reaction is a common technique [8, 9, 18, 19] used to synthesize graft or block copolymers,however,it is not easy to prepare well-defined copolymers,because the existence of b- hydrogen on the polypropylene chain can result in side reactions, such as chain scission and crosslinking. Furthermore,the high molecular weight and crystallization of terminated functional iPP also result in a reduced reactivity. Although ionic polymerization [11, 20] and controlled/living free radical techniques [21, 22] are mostly employed in the functionalization of iPP in order to get well-defined copolymer structure. Both reactions mentioned above need strict conditions,such ascomplete absence of moisture and other acidic impurities,which impede their industrialization applications. Furthermore,there are few transition metal coordi- nation catalysts that exhibit living polymerization behavior,and most of them are limited to the preparation of polyethylene and poly(1-hexene) [5, 23, 24, 25, 26].
In recent years,the coupling reaction,one of the most significant but non-mainstream methods,was introduced in PP functionalization to produce novel PP copolymer structures. Soga et al. [12] successfully introduced the coupling reaction into the functionalization of PP. They synthesized the Zn-terminated PP by the TiCl3/Al(C2H5)2Cl catalyst system using ZnEt2 as chain transfer reagent. Then the Zn-terminated PP was transformed into vinyl- terminated PP by a coupling reaction. Lu [10] prepared long chain branched PP with a relatively well-defined molecular structure using a coupling reaction,whose backbone molecular weight,graft length,and graft density can be well controlled by modulating the ratio between PP-g-maleic anhydride and PP-t-NH2. The metal catalyzed azide/alkyne ‘click’ reaction represents an important contribution to the functionalization of iPP due to its high reactivity. Huang et al. [27] synthesized a star iPP by coupling the azide-terminated-iPP with the trialkyne-containing multifunc- tional compound. Dong et al. [28] fabricated azide and alkyne functionaliPP,withazideatthechainendofiPPwhilealkyneatthe side chain,and then coupled them effectively by click chemistry. From previous works one conclusion can be drawn that the key to coupling reaction applied to polypropylene is to maximize the reactive activity because the reaction agent used is of low reaction activity due to the large spacial steric hindrance and heteroge- neous reaction.
In this paper,we report an efficient method to synthesize iPP-t- OH and the subsequent iPP-b-PEG copolymers by a combination of coordinationpolymerizationandcouplingreaction.TheiPP-b-PEGis anexcellentcompatilizerforpolypropylenematrixcompositeswith polarfillerorotherpolarpolymers.Furthermore,itwasproposedfor useincoatingapplicationsthattheiPP-b-PEGcanbeemployedasan interlayer,or prime coat,between the polar substrate and a polypropylene topcoat. The coordination polymerization was catalyzed by TiCl4/MgCl2/AlEt3 catalyst system and ZnEt2 as chain transfer agent,since MgCl2-supported Ziegler-Natta catalysts are playing major roles inthe production of polyolefins,including more than50milliontonsofPPeachyear.TheresultantZn-terminatedPP was oxidized and hydrolyzed toprovidea monohydroxyl-terminat- ed PP (iPP-t-OH). Then iPP-t-OH was successfully reacted with isocyanate-terminated poly(ethylene glycol) (PEG-t-NCO) to pre- pare di-block copolymer containing the iPP segment via coupling reaction due to high reactivity between -OH and -NCO. Thus,a simple but highly-efficient method for preparation of di-block copolymer containing iPP segment was achieved.
2. Experimental 2.1. Materials and equipmentsTiCl4/MgCl2/diester catalyst was kindly donated by SINOPEC Catalyst Co.,Ltd.,Beijing,China. The catalyst had a Ti content of 2.7 wt%. Triethylaluminium (TEA) was purchased from Albemarle. Cyclohexyl(dimethoxy)methylsilane(C-donor)waspurchasedfrom Huabang Chemistry Ltd.,Hubei,China. Diethylzinc (15 wt% in toluene) was purchased from Aldrich. Propylene (purity >99.9%) andO2(purity>99.5%)werepurchasedfromHangzhouMinxingGas Co.,Ltd.,China. Toluene,acetone,monohydroxyl-terminated poly(ethylene glycol) (PEG-t-OH,Mn= 2000 g/mol,Mw/Mn= 1.1), dichloromethane,dibutyltin dilaurate (DBTDL),3-isocyanato- methyl-3,5,5-trimethylcyclohexyl isocyanate (IPDI, purity >99.5%),hydrochloric acid were purchased from Aldrich. Toluene waspurified oversodium/benzophenone ketyland distilled undera dryN2atmospherepriortouse.PEGwasdistilledintoluenefor6 hto remove water before reaction. Extra-pure-grade (99.99%) nitrogen was further purified by passing through two columns of de-oxygen catalysts and pre-activated 4 A˚ molecular sieves to remove the residualmoistureandoxygen.Allmoisture-sensitivemanipulations were carried out through Schlenk technique.
Fourier transform infrared spectroscopy was performed on a Bruker Vector 22 FT-IR spectrometer (Germany). DSC measure- ment was carried out on a Q100 MDSC (TA Instruments Corporation,USA) under a nitrogen flow of 50 mL/min. It was operatedfrom?20 ℃to200 ℃ataheating/coolingrateof10 ℃/min. Molecular weight and polydispersity index were measured with gel permeation chromatography on a PL-GPC220 at 120 ℃ using 1,2,4-trichlorobenzene as eluent and polystyrene as standard. The1H NMR measurement of PEG-t-NCO was conducted on a Varian-Inova-500 HZ spectrometer using deuterated chloroform (CDCl3) as solvent. The other1H NMR and13C NMR spectra were performed on a Varian Mercury 300 Plus instrument in the pulse Fourier mode and recorded with deuterated 1,2-dichlorobenzene at120 ℃.Inthecaseof13CNMR,theparameterofpulserepetition and scanning times is set to 20 s and 8000 s,respectively,for the purpose of getting high resolution spectra.
2.2. Synthesis of hydroxyl-terminated iPPIn a typical propylene coordination polymerization,50 mL toluene,a specific amount of triethylaluminium (Al/Ti = 100),C- donor (Si/Ti = 5) and ZnEt2 (Zn/Ti = 3-40) were injected into a 100 mL Schlenk flask under propylene atmosphere at 1 atm. About 40 mg of TiCl4/MgCl2catalyst was added into the mixed solutionto initiate the polymerization at 60 ℃. 30 min later,the reaction mixture was heated to 100 ℃ and contacted with dry oxygen gas for another 30 min. Finally,the reaction was quenching by hydrochloride acidified methanol. The solvent was removed by rotary evaporator and the polymer was washed with acetone- water mixture and dried in vacuum at 60 ℃ for 24 h. With the purpose of determining the influence of chain transfer on the isotactic index (II) of iPP-t-OH,a series of Soxhlet extraction experiments were performed at 100 ℃ (near the boiling point of n- heptane) for 24 h. The isotactic index (II) was defined as the weight percentage of n-heptane insoluble fraction in the sample. Both the n-heptane soluble fraction and n-heptane insoluble fraction were evaporated and then dried in vacuum at 60 ℃ for 24 h.
2.3. Synthesis of isocyanate-terminated PEGDBTDL (20 mg [1 wt% (PEG-t-OH)]),IPDI (0.222 g,1 mmol) and 5 mL CH2Cl2were added into a 100 mL Schlenk flask at 55 ℃. Then 2 g (1 mmol) PEG-t-OH dissolved in 50 mL dichloromethane was injected into the 100 mL Schlenk flask slowly by a micro injection pump at a rate of 0.2 mL/min. Then,the mixture was refluxed at 55 ℃ for 4 h. Finally,the mixture solution was precipitated by n- heptane and dissolved in dichloromethane repeatedly to remove impurities and then dried in vacuum at 30 ℃ for 24 h. PEG-t-NCO (2.09 g,Mn= 2200 g/mol,Mw/Mn= 1.03) was obtained.
2.4. Synthesis of iPP-b-PEG via coupling reactionPEG-t-NCO (1.32 g,0.6 mmol),0.5 mmol iPP-t-OH (entry 2,3,5 and 7 in Table 1) and 50 mL toluene were added into a 100 mL Schlenk flask and stirred for 1 h at 110 ℃. Subsequently,1 wt% (iPP-t-OH) of DBTDL was added into the Schlenk flask and the mixture was stirred for 8 h. Finally the mixture solution was precipitated by n-heptane and dissolved in dichloromethane repeatedly to remove impurities and dried in vacuum at 60 ℃ for 24 h.
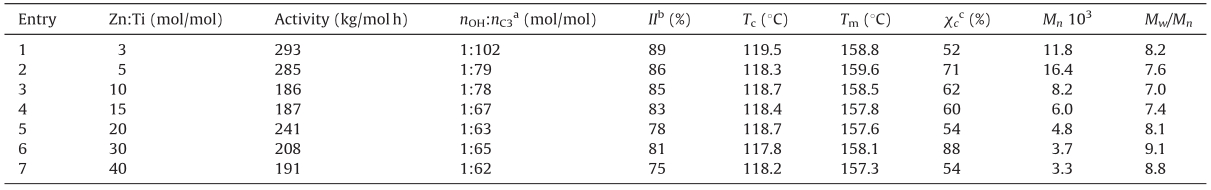
| Table 1 Summary of the propylene polymerization. |
A route to prepare iPP-t-OH and the subsequent iPP-b-PEG is illustrated in Scheme 1. ZnEt2 is chosen as chain transfer agent because the Zn-terminated PP can be transformed to various end chainfunctionalpolymersorblockcopolymers[12, 29].IPDIserved as coupling agent during the subsequent coupling reaction.
3.1. Synthesis of iPP-t-OHiPP-t-OH was synthesized by simple coordination polymeriza- tion catalyzed by TiCl4/MgCl2 catalyst and using ZnEt2 as chain transfer agent,which has potential to be industrialized. The polymerization results are summarized in Table 1.

|
Download:
|
| Scheme 1. Synthetic routes for iPP-t-OH and iPP-b-PEG. | |
AsshowninTable1,withtheincreaseofZnEt2 consumption,the polymerization activity tends to decrease to some extent. It is a reflection of the competitive coordination at the propagating Ti active site between monomer and chain transfer agent,and the changeintheconsumptionofZnEt2 doesnotinfluencethestructure of PP significantly because those parameters relating to the structure,such as isotacticity (II),crystallization point (Tc),melting point (Tm) and crystallinity (xc),are unchanged. However,the molecular weight of the resultant PP decreases significantly. Namely,using ZnEt2 as chain transfer agent,the molecular weight of iPP-t-OH could be regulated in a wide range without influencing the chain structure and macroscopic performances. With TiCl3/ AlEt2Cl catalyst system,Sogaetal.have prepared iPP that exhibiteda melting point at around 150 ℃ with a shoulder at approximately 158 ℃ [30, 31]. However,in this work when using TiCl4/MgCl2/ diestercatalystsystem,aniPPwithTm> 157 ℃isobtained.Namely, the isospecificity of the former is lower than that of the latter.
Furthermore,the 13C NMR spectrum can also be used to characterize the stereoregularity and the distinguishable reso- nance peaks can be attributed to the pentad configuration sequences completely [33]. The 13C NMR spectrum of a typical iPP-t-OH(entry 5 in Table 1) isshown in Fig. 1a. To characterize the isotacticity of this sample,the region located at 19-22 ppm (the resonance peaks of the methyl group) is focused on and shown as Fig. 1b because it could provide high resolution information for stereoregularity [34]. Methyl spectra and the triad assignments were made previously [33, 35] and unequivocally. The fine structure in each region,in most cases resolved to the base line, is assigned to pentad sequences as showed in Fig. 1b. According to the13C NMR spectrum,the calculated isotacticity of this sample was 83% by the fraction of pentad sequences ([mm]%). Unfortu- nately,the resonance peaks of methylene connected with the terminated hydroxyl groups could not be found because of high molecular weight and low content of hydroxyl group in iPP-t-OH. However,the existence of terminated hydroxyl groups could be proved by the other sensitive measurements,such as FT-IR and 1H NMR. The further chain extended reaction would also provide reliable evidence for the successful synthesis of iPP-t-OH.

|
Download:
|
| Fig. 1.125 MHz13C NMR spectra of a typical iPP-t-OH (entry 5 in Table 1): (a) the complete spectrum; (b) the methyl resonance region (solvent,C2D2Cl4; temp.,120 ℃). | |
The productsof coordination polymerizations are characterized by FT-IR. As shown in Fig. 2,hydroxyl group is successfully introduced to the end of iPP chain,as proven by a stretching frequency at 3440 cm?1in the FT-IR spectra,which is assigned to hydroxyl group. And as the consumption of ZnEt2 increases,those peaks at 3440 cm?1become stronger and stronger. Furthermore, these products are also characterized by1H NMR. As shown in Fig. 3a,three peaks at 0.75-1.7 ppm correspond to -CH3,-CH2and -CH on iPP backbone,respectively. Two sets of additional weak chemical shifts at 3.1-3.5 ppm,corresponding to -CH(CH3)-CH2- OH methylene proton resonance located in a diastereotopic environment,indicate the existence of hydroxyl groups at the terminal of iPP [36, 37]. From the1H NMR results,the content of hydroxyl groups in every product is calculated and shown in Table 1. Obviously,the content of hydroxyl group in the products increases gradually as the consumption of ZnEt2 increased. As shown in Fig. 3a,there is no peak from vinyl groups. It indicates that termination by b-H elimination is negligible and ZnEt2 chain transfer reaction dominates the chain-termination pathway [4].

|
Download:
|
| Fig. 2. Representative FT-IR spectra of iPP-t-OH in Table 1. | |
Those iPP-t-OH samples corresponding to entry 2,3,5 and 7 in Table 1 were combined with PEG-t-NCO by coupling reaction,respectively. Therefore,a series of di-block copolymers containing iPP segment (iPP-b-PEG) was prepared. The yield of these reactions is higher than 80%. In order to further confirm the structure of iPP- b-PEG,a1H NMR spectrum of intermediates,PEG-t-NCO,was also recorded and shown in Fig. 3b,and the characteristic peaks were also designated. In Fig. 3b,the sharp peaks centered at 3.65 ppm and 3.38 ppm can be attributed to methylene protons of -CH2CH2O- and -OCH3end groups in monomer PEG,respectively. Thevery weakpeakat4.20 ppmisattributedtomethyleneprotons of-CH2-COO-inPEGendthatlinkedwithIPDI.Thecomplexpeaks centered at 1 ppm are attributed to the -CH3end group and -CH2- in IPDI,respectively [38]. A typical1H NMR spectrum of iPP-b-PEG is shown in top of Fig. 3c. Those peaks at 3.1-3.5 ppm almost disappear,at the same time,a strong peak which can be attributed to -CH2O- in PEG appears at 3.7-3.8 ppm. Moreover,a weak peak centered at 4.37 ppm comes from the newly formed -CH2-COO- groups generated in the coupling reaction step. It shows that those hydroxyl groups at the chain end of iPP are almost consumed, indicatingthatthe highreactivity ofthe couplingreactionbetween -OH and -NCO.

|
Download:
|
| Fig. 3. 1H NMRspectraof(a)iPP-t-OH(entry2,3,5,7respectivelyinTable1),(b)iPP-t-NCOfromentry5inTable1,and(c)iPP-b-PEGfromentry5inTable1(top)andiPP-t-OH (entry 5 in Table 1) (bottom). | |
The DSC results of iPP-t-OH and iPP-b-PEG are shown in Fig. 4. Themelting point(Tm) of iPP-t-OH isaround 160 ℃ (Fig.4a),butTm of iPP-b-PEG is around 137 ℃ (Fig. 4b). The crystallization points (Tc) of the former and the latter are about 120 ℃ and 107 ℃, respectively. The remarkable changes in Tmand Tcsuggest that the crystallization process of iPP is influenced by some factors. The existenceof PEG segment would havea significantinfluence onthe crystal parameters,such as crystal form and lamellar thickness of iPP,indicating that PEG was successfully connected with iPP.Evidence that,iPP-b-PEG was prepared by a convenient,highly- efficient method.

|
Download:
|
| Fig. 4. DSC curves of (a) iPP-t-OH (entry 5 in Table 1) and (b) corresponding iPP-b- PEG. | |
The hydroxyl-terminated isotactic polypropylene (iPP-t-OH) can be prepared by coordination polymerization (catalyzed by MgCl2-supported Ziegler-Natta catalyst using ZnEt2 as chain transfer agent) and the subsequent oxidation and hydrolysis. The introduction of ZnEt2 does not influence the chain structure and macroscopic performances of iPP segment significantly,but can regulate the molecular weight of iPP segment effectively. The reactivitybetween-OHand-NCOisveryhigh.Usingdi-isocyanate as coupling agent,di-block copolymer containing iPP segment, such as iPP-b-PEG,can be prepared efficiently. Therefore,a convenient,highly-efficient method to prepare other high-perfor- mance di-block copolymers by linking the terminated -OH or - NCO with iPP-t-OH is developed. Further design and synthesis of the other special functional di-block copolymers containing iPP segment are currently underway.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 51173157),National High-Tech R&D Program of China (No. 2012AA040305) and the Major State Basic Research Programs (No. 2011CB606001).
| [1] | T.C.M. Chung, Functionalization of Polyolefins, Academic Press Inc., Califoria, 2002. |
| [2] | L.S. Boffa, B.M. Novak, Copolymerization of polar monomers with olefins using transition-metal complexes, Chem. Rev. 100 (2000) 1479-1493. |
| [3] | R. Mülhaupt, Catalytic polymerization and post polymerization catalysis fifty years after the discovery of Ziegler’s catalysts, Macromol. Chem. Phys. 204 (2003) 289-327. |
| [4] | S.B. Amin, T.J. Marks, Versatile pathways for in situ polyolefin functionalization with heteroatoms: catalytic chain transfer, Angew. Chem. Int. Ed. 47 (2008) 2006- 2025. |
| [5] | W.T. Lin, J.Y. Dong, T.C.M. Chung, Synthesis of chain end functional isotactic polypropylene by the combination of metallocene/MAO catalyst and organoborane chain transfer agent, Macromolecules 41 (2008) 8452-8457. |
| [6] | T.C. Chung, Synthesis of functional polyolefin copolymers with graft and block structures, Prog. Polym. Sci. 27 (2002) 39-85. |
| [7] | E.J. Goethals, Telechelic Polymers: Synthesis and Applications, CRC Press Inc., Florida, 1989. |
| [8] | B. Lu, T.C. Chung, Maleic anhydride modified polypropylene with controllable molecular structure: new synthetic route via borane-terminated polypropylene, Macromolecules 31 (1998) 5943-5946. |
| [9] | B. Lu, T.C. Chung, New maleic anhydride modified PP copolymers with block structure: synthesis and application in PP/polyamide reactive blends, Macromolecules 32 (1999) 2525-2533. |
| [10] | B. Lu, T.C. Chung, Synthesis of long chain branched polypropylene with relatively well-defined molecular structure, Macromolecules 32 (1999) 8678-8680. |
| [11] | A.M. Anderson-Wile, G.W. Coates, F. Auriemma, C. De Rosa, A. Silvestre, Synthesis and ring-opening metathesis polymerization of norbornene-terminated syndiotactic polypropylene, Macromolecules 45 (2012) 7863-7877. |
| [12] | H. Kurosawa, T. Shiono, K. Soga, Synthesis of vinyl-terminated isotactic poly(propylene) using the coupling reaction between Zn-terminated polymer and allyl halides, Macromol. Chem. Phys. 195 (1994) 1381-1388. |
| [13] | T. Shiono, K. Soga, Synthesis of terminally aluminum-functionalized polypropylene,Macromolecules 25 (1992) 3356-3361. |
| [14] | T. Shiono, K. Soga, Synthesis of terminally halogenated isotactic poly(propylene)s using hydroalumination, Makromol. Chem. Rapid Commun. 13 (1992) 371-376. |
| [15] | T. Shiono, K.K. Kang, H. Hagihara, T. Ikeda, Novelty of vinylidene-terminated polypropylene prepared by a MgCl2-supported TiCl4 catalyst combined with AlEt3 as cocatalyst, Macromolecules 30 (1997) 5997-6000. |
| [16] | W.Q. Weng, E.J. Markel, A.H. Dekmezian, Synthesis of vinyl-terminated isotactic poly(propylene), Macromol. Rapid Commun. 21 (2000) 1103-1107. |
| [17] | S.G. Gaynor, Vinyl chloride as a chain transfer agent in olefin polymerizations: preparation of highly branched and end functional polyolefins, Macromolecules 36 (2003) 4692-4698. |
| [18] | T.C. Chung, D. Rhubright, G. Jiang, Synthesis of polypropylene-graft-poly(methyl methacrylate) copolymers by the borane approach, Macromolecules 26 (1993) 3467-3471. |
| [19] | T.C. Chung, D. Rhubright, Polypropylene-graft-polycaprolactone: synthesis and applications in polymer blends, Macromolecules 27 (1994) 1313-1319. |
| [20] | J.H. Song, B. Messer, Y.Y. Wu, H. Kind, P.D. Yang, MMo3Se3(M = Li+, Na+, Rb+, Cs+, NMe4 +) nanowire formation via cation exchange in organic solution, J. Am. Chem. Soc. 123 (2001) 9714-9715. |
| [21] | K. Matyjaszewski, J. Saget, J. Pyun, M. Schlö gl, B. Rieger, Synthesis of polypropylene- poly(meth)acrylate block copolymers using metallocene catalyzed processes and subsequent atom transfer radical polymerization, J. Macromol. Sci. A 39 (2002) 901-913. |
| [22] | H. Kaneko, J. Saito, N. Kawahara, et al., Synthesis and characterization of polypropylene- based block copolymers possessing polar segments via controlled radical polymerization, J Polym. Sci. A: Polym. Chem. 47 (2009) 812-823. |
| [23] | H. Yasuda, M. Furo, H. Yamamoto, et al., New approach to block copolymerizations of ethylene with alkyl methacrylates and lactones by unique catalysis with organolanthanide complexes, Macromolecules 25 (1992) 5115-5116. |
| [24] | M. Brookhart, J. DeSimone, B.E. Grant, M.J. Tanner, Cobalt(ⅡI)-catalyzed living polymerization of ethylene: routes to end-capped polyethylene with a narrow molar mass distribution, Macromolecules 28 (1995) 5378-5380. |
| [25] | K.J. Shea, J.W. Walker, H. Zhu, M. Paz, J. Greaves, Polyhomologation. A living polymethylene synthesis, J. Am. Chem. Soc. 119 (1997) 9049-9050. |
| [26] | G. Desurmont, T. Tokimitsu, H. Yasuda, First controlled block copolymerizations of higher 1-olefins with polar monomers using metallocene type single component lanthanide initiators, Macromolecules 33 (2000) 7679-7681. |
| [27] | H.H. Huang, H. Niu, J.Y. Dong, Synthesis of star isotactic polypropylene using click chemistry, Macromolecules 43 (2010) 8331-8335. |
| [28] | C.H. Zhang, H. Niu, J.Y. Dong, Facile functionalization of isotactic polypropylene by azide and alkyne groups for click chemistry application, Appl. Organomet. Chem. 25 (2011) 632-637. |
| [29] | T. Shiono, H. Kurosawa, K. Soga, Isospecific polymerization of propene over TiCl3 combined with bis(.omega.-alkenyl) zinc compounds, Macromolecules 28 (1995) 437-443. |
| [30] | T. Shiono, K. Yoshida, K. Soga, Synthesis of terminally hydroxylated isotactic polypropylene using Zn(C2H5)2 and oxygen as chain transfer and quenching reagents, Makromol. Chem. Rapid Commun. 11 (1990) 169-175. |
| [31] | L.F. Tong, Y. Shen, Q. Zheng, Y.G. Shang-guan, A novel approach for observing morphology of polypropylene under ultrasonic vibration by SALS, Chin. Chem. Lett. 15 (2004) 841-844. |
| [32] | J.E. Mark, Polymer Data Handbook, Oxford University Press, New York, 1999. |
| [33] | A. Zambelli, P. Locatelli, G. Bajo, F.A. Bovey, Model compounds and 13C NMR observation of stereosequences of polypropylene, Macromolecules 8 (1975) 687- 689. |
| [34] | A. Zambelli, D.E. Dorman, A.I.R. Brewster, F.A. Bovey, Carbon-13 observations of the stereochemical configuration of polypropylene, Macromolecules 6 (1973) 925-926. |
| [35] | Y. Inoue, A. Nishioka, R. Chuûjoô, Carbon-13 nuclear magnetic resonance spectroscopy of polypropylene, Die Makromol. Chem. 152 (1972) 15-26. |
| [36] | F.Y. Tzeng, M.C. Lin, J.Y. Wu, et al., Stereoregular diblock copolymers of syndiotactic polypropylene and polyesters: syntheses and self-assembled nanostructures, Macromolecules 42 (2009) 3073-3085. |
| [37] | J.Y. Dong, G.Q. Fang, Z.C. Han, Hydroxyl-terminated polypropylene and its preparation method, Chinese Patent 20041000990.6, 2004. |
| [38] | C. Gong, Z. Qian, C. Liu, et al., A thermosensitive hydrogel based on biodegradable amphiphilic poly(ethylene glycol)-polycaprolactone-poly(ethylene glycol) block copolymers, Smart Mater. Struct. 16 (2007) 927-933. |