b Department of Chemistry, Faculty of Science, University of Shahid Beheshti, Tehran 19835389, Iran;
c Department of Chemistry, Faculty of Science, Arak Branch, Islamic Azad University, Arak, Iran
The therapeutic and pharmaceutical features of sulfonamides have been reported for many years. These derivatives were intensively investigated as the first effective antibacterial agents [1]. Sulfonamides are still widely used for conditions such as acne and urinary tract infections caused by bacteria resistant to other antibiotics [2]. Sulfonamides can be carbonic anhydrase inhibitors, diuretic and hypoglycemic reagents,and pharmaceutical agents for the treatment of different diseases such as infections, Alzheimer,HIV,and cancer [3, 4, 5]. Among N-containing heterocyclic compounds,phthalazine has attracted scientific interest. Phthalazine derivatives were found to possess multiple biological activities such as antimicrobial,anticonvulsant,antifungal,anticancer, and anti-inflammatory activities [6, 7, 8]. Phthalazine derivatives synthesis provides an entrance to a variety of compounds with pharmacological activities. Following our experiences in electrochemical synthesis of sulfonamides based on the in situ generation of Michael acceptors [9, 10, 11],we envisioned that organic compounds containing both phthalazine and sulfonamide moieties might possess enhanced pharmaceutical properties and medicinal activities. This idea prompted us to investigate the electrochemical oxidation of 2,3-dihydrophthalazine-1,4-dione (1) in the presence of p-toluenesulfinic acid (2a) and benzensulfinic acid (2b) as nucleophiles and we have reported an easy and onepot electrochemical method for the synthesis of sulfonamide derivatives (3a and 3b) in good yields and purity,using environmentally friendly protocols with high atom economy.
Reaction equipment was described in an earlier paper [12]. 2,3- Dihydrophthalazine-1,4-dione,toluene and benzene derivatives of sulfinic acid were obtained from commercial sources. A solution of phosphate buffer (60 mL,c = 0.2 mol/L,pH 2.0) in a water/ acetonitrile (85/15%,v/v) solution containing 2,3-dihydrophthalazine- 1,4-dione (1) (0.25 mmol) and 4-toluenesulfinic acid (or benzensulfinic acid) (0.25 mmol) was electrolyzed in a divided cell equipped with a carbon anode (an assembly of four rods) and a large stainless steel gauze as the cathode,at 0.85 V vs. Ag/AgCl,at 25 8C. The electrolysis was terminated when the current decreased by more than 95%. The process was interrupted several times during the electrolysis and the carbon anode was washed in acetone in order to reactivate the anode. At the end of the electrolysis,the cell was placed in a refrigerator overnight. The precipitated solid was collected by filtration and was washed several times with water. After drying,the products were characterized by IR,NMR (1H and 13C) and MS.
2,3-Dihydro-2-tosylphthalazine-1,4-dione (C15H12N2O4S) (3a): Isolated yield: 75%. Mp. >270 8C (dec.),yellow. IR (KBr,cm-1): n 2512,1738,1672,1495,1298,1255,1208,1115,1073,1022,825, 784,679,618,559,380,230. 1HNMR(300 MHz,DMSO-d6): d 1.7 (s, 3H),6.8 (d,2H),7.5 (d,2H),7.8 (d,2H),8.1 (d,2H),11.9 (NH,1H). 13C NMR (75 MHz,DMSO-d6): d 24.2,120.1,126.4,126.5,128.4, 128.5,130.1,135.6,136.7,153.1,157.2. MS (m/z) (relative intensity): 317 [M+H]˙+ (80),252 (50),221 (25),163 (100),132 (25),104 (75),76 (35),50 (20).
2-(Phenylsulfonyl)-2,3-dihydrophthalazine-1,4-dione (C14H10N2O4S) (3b): Isolated yield: 70%. Mp. >270 8C (dec.), yellow. IR (KBr,cm-1): n 2906,1659,1493,1335,1308,1241, 1208,1083,1030,824,778,672,619,486,440. 1H NMR (300 MHz, DMSO-d6): d 6.9 (d,2H),7.9 (m,3H),7.8 (d,2H),8.3 (d,2H) 10.7 (NH,1H). 13C NMR (75 MHz,DMSO-d6): d 120.1,126.3,128.2, 128.5,128.7,130.7,131.1,134,156.1,163.1. MS (m/z) (relative intensity): 303 [M+H]˙+ (35),239 (30),221 (100),163 (100),132 (20),104 (60),76 (35),50 (20).
Cyclic voltammogram of a 1.0 mmol/L solution of 2,3-dihydrophthalazine- 1,4-dione (1) in a water (0.2 mol/L phosphate buffer,pH 2.0)/acetonitrile (85/15%,v/v) is shown in Fig. 1,curve a. As can be seen,one anodic (A1) and a cathodic peak C1 were obtained at 1.0 and 0.78 V (E1/2 = 0.89 V) versus Ag/AgCl. The anodic and cathodic peaks are counterpart and correspond to the transformation of 1 to phthalazine-1,4-dione (1ox) and vice versa within a quasi-reversible two-electron process. The peak current ratio (IpC1/IpA1) is less than unity and decreases with the reduction of the potential sweep rate indicating that the generated phthalazine-1,4-dione (1ox) is not stable. This instability is related to the oxidative ring cleavage of 1,as discussed in details in our previously published paper [12]. The oxidation of 1 in the presence of p-toluenesulfinic acid (2a) as nucleophile was studied using cyclic voltammetry. Fig. 1,curve b,shows the cyclic voltammogram obtained for a 1.0 mmol/L solution of 1 in the presence of 1.0 mmol/L of 2a. It is clear that the cathodic peak (C1) disappeared and a new anodic peak (A2) appeared with a more positive potential. In this Figure,the cyclic voltammogram of 2a is shown in curve c. A close analysis of these three voltammograms (curves a- c) suggested that A2 corresponds to 1ox,which is bonded to 2a. A similar observation was made in the case of 1 in the presence of 2b.

|
Download:
|
| Fig. 1.A cyclic voltammogram of 1 (1.0 mmol L-1) in the absence of 2a (a),in the presence of 2a (1.0 mmol L-1) (b),2a (1.0 mmol L-1) in the absence of 1,in a water/ acetonitrile (85/15%,v/v) solution containing phosphate buffer (c = 0.2 mol L-1,pH 2.0) (c). Scan rate: 800 mV s-1,temperature = (25 ± 1) 8C. | |
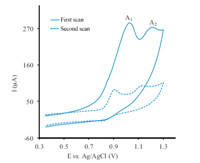
The multi-cyclic voltammograms of 1.0 mmol/L 1 in the presence of 1.0 mmol/L 2a are shown in Fig. 2. The reduced height of the anodic peak (A1) in the second scan is probably due to the formation of a thin film of product at the surface of the electrode, inhibiting to a certain degree the performance of the electrode process that was enhanced during the repetitive cycling of the potential.
|
Download:
|
| Fig. 2.Multi-cyclic voltammograms of 1 (1.0 mmol/L) in the presence of 2a (1.0 mmol/L) at glassy carbon electrode in an aqueous phosphate buffer (0.2 mol L-1,pH 2.0)/acetonitrile (85/15,v/v) solution. Scan rate: 800 mV s-1, temperature = (25 ± 1) 8C. | |
Controlled-potential coulometry was performed in water (0.2 mol/L phosphate buffer,pH 2.0)/acetonitrile (85/15,v/v) containing 0.25 mmol of 1 and 0.25 mmol of 2a at 0.85 V versus Ag/AgCl. The electrolysis progress was monitored using cyclic voltammetry (Fig. 3). It was found that,proportional to the advancement of electrolysis,the anodic peak A1 decreased.

|
Download:
|
| Fig. 3.Cyclic voltammograms of 1 (0.25 mmol) in the presence of 2a (0.25 mmol),at a glassy carbon electrode in an aqueous phosphate buffer (0.2 mol L-1,pH 2.0)/ acetonitrile (85/15,v/v) solution during controlled-potential coulometry at 0.85 V versus Ag/AgCl. Scan rate: 100 mV s-1,temperature = (25 ± 1) 8C. | |
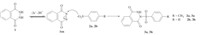
Diagnostic criteria of cyclic voltammetry,accompanied by spectroscopic data (IR,1H NMR,13C NMR and MS) of the final products allow us to propose the mechanistic pathway in Scheme 1 for the electrochemical oxidation of 1 in the presence of 2a and 2b. According to our results,it seems that a 1,4-Michael type addition reaction of 2a and 2b with 1ox is faster than other secondary reactions,leading to the formation of 3a and 3b. The oxidation of these compounds (3a and 3b) is harder than the oxidation of the starting molecule (1) by virtue of the presence of an electronwithdrawing phenylsulfonyl group on 3a and 3b.
|
Download:
|
| Scheme 1.Proposed mechanism for the electrochemical oxidation of 1 in the presence of arylsulfinic acids (2a and 2b). | |
The results of this work show that 2,3-dihydrophthalazine-1,4- dione (1) is oxidized to phthalazine-1,4-dione (1ox),which is then attacked by arylsulfinic acids (2a and 2b). The final products are obtained via an EC mechanism after a Michael type addition of arylsulfinic acids to the electro-generated 1ox. According to our results,these processes lead to the formation of new sulfonamide derivatives in good yields. The presented work represents a facile and reagent-less method with high atom economy for the synthesis of sulfonamides using a carbon electrode.
We acknowledge Bu-Ali Sina University Research Council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for their support of this work.
| [1] | Z. Huang, Z. Lin, J. Huang, A novel kind of antitumour drugs using sulfonamide as parent compound, Eur. J. Med. Chem. 36 (2001) 863-872. |
| [2] | S.F. Yang, C.F. Lin, C.J. Wu, K.K. Ng, A.Y.C. Lin, P.K.A. Hong, Fate of sulfonamide antibiotics in contact with activated sludge—sorption and biodegradation, Water Res. 46 (2012) 1301-1308. |
| [3] | K. Seri, K. Sanai, K. Kurashima, Y. Imamura, H. Akita, (R)-ACX is a novel sufonylurea compound with potent, quick and short-lasting hypoglycemic activity, Eur. J. Pharmacol. 389 (2000) 253-256. |
| [4] | S. Bano, J. Javed, S. Ahmad, I.G. Rathish, S. Singh, M.S. Alam, Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents, Eur. J. Med. Chem. 46 (2011) 5763-5768. |
| [5] | X.P. Hong, J.Y. Ma, Electrochemical study of sulfadiazine on a novel phthalocyanine- containing chemically modified electrode, Chin. Chem. Lett. 24 (2013) 329- 331. |
| [6] | R. Sivakumar, S.K. Gnanasam, S. Ramachandran, Pharmacological evaluation of some new 1-substituted-4-hydroxy-phthalazines, Eur. J. Med. Chem. 37 (2002) 79-8013. |
| [7] | S.L. Zhang, Y.J. Liu, Y.F. Zhao, Q.T. Guo, P. Gong, Synthesis and antitumor activities of novel 1,4-substituted phthalazine derivatives, Chin. Chem. Lett. 21 (2010) 1071-1074. |
| [8] | X. Zhai, J. Li, L. He, S. Zheng, Y.B. Zhang, P. Gong, Synthesis and in vitro cytotoxicity of novel 1,4-disubstituted phthalazines, Chin. Chem. Lett. 19 (2008) 29-32. |
| [9] | D. Nematollahi, A. Maleki, Electrochemical oxidaton of N,N-dialkyl-π-πhenylenediaminesin the presence of arylsulfinic acids. An efficient method for the synthesisof new sulfonamide derivatives, Electrochem. Commun. 11 (2009) 488-491. |
| [10] | D. Nematollahi, E. Mehdipour, A. Zeinodini-Meimand, A. Maleki, Chemical and electrochemical oxidative coupling of N,N-dialkyl-π-πhenylenediamines and arylsulfinic acids. Synthesis of sulfonamide derivatives, Tetrahedron Lett. 51 (2010) 6447-6450. |
| [11] | F. Varmaghani, D. Nematollahi, S.E. Mallakpour, R. Esmaili, Electrochemical oxidation of 4-substituted urazoles in the presence of arylsulfinic acids: an efficient method for the synthesis of new sulfonamide derivatives, Green Chem. 14 (2012) 963-967. |
| [12] | F. Varmaghani, D. Nematollahi, Electrochemical study of 1,2-dihydropyridazine- 3,6-dione in protic and aprotic solvents: oxidative ring cleavage and reduction, Electrochim. Acta 56 (2011) 6089-6160. |




