b School of Chemistry, Liaocheng University, Liaocheng 252059, China
Organotin compounds show potential applications ranging from a variety of biological activity to industrial catalysts,many of which have been reported to exhibit potent anticancer activity [1]. Terpyridine derivatives are remarkable ligands for almost all the transition metal ions through the {N,N,N} coordination mode, some of their complexes were even evaluated as antitumor drugs [2]. Except for some Mo¨ssbauer spectra and the crystal data for the simple tin complexes of terpyridine that were published [3, 4, 5, 6], however,little research has been carried out on their antitumor ability.
In this report,two terpyridine derivatives were synthesized and coordinated with various Sn(IV) precursors. 40-p-9-Anthracenevinyl- 2,20:6,200-terpyridine (ANTPY) possesses a large conjunction system and shows strong fluorescence emission,which should be extremely attractive for studying the absorption,accumulation, degradation of the related organotin compounds in aquatic organisms and imaging of chemical species in biological tissues using confocal microscopy [7]. 40-p-N,N-Bis(2-hydroxyethyl)benzyl- 2,20:6,200-terpyridine (TPYOH) with two 2-hydroxyethyl groups is highly soluble in water,which is designed to introduce hydrogen bonding interactions with the biological targets. We reported here the structural characterization of five terpyridine-Sn(IV) complexes,together with their fluorescent ability and preliminary test for in vitro cytotoxicity.
4-p-Bromomethylphenyl-2,20:6,200-terpyridine was prepared according to the published procedures [8]. p-N,N-Bis(2-hydroxyethyl) amino benzaldehyde was prepared as reported [9]. All organotin precursors were purchased from Sigma Chemical Co. The ESI-MS was recorded using a LCQ electron spray mass spectrometer (ESI-MS,Finnigan). The 1H NMR data were collected on a 500 MHzBrukerDMXspectrometer. The fluorescence spectra were recorded using a PerkinElmer LS55 luminescence spectrometer. The in vitro cytotoxicity was tested using the MTT assay against two tumor cell lines [10] (Fig. 1).
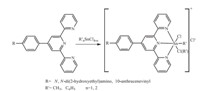
|
Download:
|
| Fig. 1.The synthesis route of terpyridine-Sn(IV) complexes. | |
40-p-9-Anthracenevinyl-2,20:6,200-terpyridine (ANTPY): 1 mmol of 4-p-bromomethylphenyl-2,20:6,200-terpyridine and 1.2 mmol of triphenylphosphine were dissolved in 30 mL of toluene. The mixture was refluxed for 3 h and then the white solid of 40-ptriphenylphosphinemethylphenyl- 2,20:6,200-terpyridine bromide (tpptpy) was collected by filtration. The solid was washed with diethyl ether three times and then dried in vacuum. Yield: 95%. 4 mmol of the sodium methoxide and 1.8 mmol of the tpptpy were dissolved in 30 mL of methanol,and then 2.8 mmol of 9-anthracenecarbaldehyde was added. The mixture was heated to 65 8C for 1 h and then the yellow precipitation of 40-p-9- anthracenevinyl-2,20:6,200-terpyridine(ANTPY) was obtained. The solid was collected by filtration and washed with ethanol,dried in vacuum. Yield: 62%. 1H NMR (500 MHz,CDCl3): d 8.98 (s,2H), 8.85 (s,2H),8.45 (s,2H),8.42 (q,2H),8.13 (t,2H),7.87 (d,4H), 7.53-7.51 (t,4H),7.07-7.04 (d,2H). ESI-MS(+p): m/z 512,534 and 1044.83 could be assigned as [ANTPY+H]+,[ANTPY+Na]+ and [(ANTPY)2+Na]+,respectively.
4'-p-N,N-Bis(2-Hydroxyethyl)benzyl-2,20:6,200-terpyridine (TPYOH): A mixture of 10.5 g (0.05 mol) of p-N,N-bis(2-hydroxyethyl) amino benzaldehyde,14.5 g (0.12 mol) of 2-acetylpyridine, 20 mL of 10 mol/L NaOH and 100 mL of ethanol were stirred vigorously at room temperature for 3 h,then cooled down to -4℃ for 1 h. The supernatant solution was decanted and the solid residue was dissolved in 200 mL of ethanol. 10 g of NaOH solid was added to the deep red solution and then 100 mL of sNH3·H2O (25%) was added dropwise. After the mixture had been refluxed for 4 h, the solvent was evaporated under high vacuum to obtain the crude product as a thick paste. 400 mL solution of ethanol and diethyl ester (1:15,v/v) was added to produce a yellow precipitate. The yellow solid was collected by filtration and dried in vacuum. Yield: 46%. 1H NMR(500 MHz,CDCl3): d 8.76 (d,2H),8.65 (d,4H),8.03 (m, 2H),7.60 (d,2H),7.52 (m,2H),6.87 (d,2H),4.86 (t,2H),3.61 (t,4H), 3.52 (t,4H). ESI-MS(+p): m/z 444.92 could be assigned as [TPYOH+CH3OH+H]+.
General synthetic procedure for the organotin compounds: 1 mmol of the terpyridine ligand was dissolved in 30 mL of methanol,then 1.2 mmol of solid organotin precursors [methyltin trichloride (MeSnCl3),dimethyltin dichloride (Me2SnCl2),benzyltin trichloride (PhSnCl3),and dibenzyltin dichloride (Ph2SnCl2), respectively] was added. The mixture immediately became dark red and was stirred under reflux for 8 h. When cooled to room temperature the tiny dark-red crystals could be obtained. The solid was collected by filtration,washed with ethanol and then dried in vacuum. 1H NMR (500 MHz,DMSO-d6) ANTPYSnMeCl3: d 8.90 (s, 2 H),8.87 (s,2H),8.45 (s,2H),8.42 (q,2H),8.16 (t,2H),7.5 (d,4H), 7.53 (t,4H),7.08 (d,2H),1.06(s,3H); TPYOHSnMeCl3: 0.93 (s,3H), 3.52 (t,4H),3.59 (m,4H),4.85 (t,2H),6.89 (d,2H),7.51 (t,2H). 7.76 (d,2H),8.01 (d,2H),8.65 (d,4H),8.77 (d,2H); TPYOHSnMe2Cl2: 1.04 (s,3H),1.06 (t,3H),3.50 (t,4H),3.60 (m,4H),4.85 (t,2H),6.87 (d,2H),7.52 (t,2H). 7.76 (d,2H),8.01 (d,2H),8.64 (d,4H),8.76 (d, 2H); TPYOHSnPhCl3: 3.52 (m,4H),3.61 (m,4H),4.86 (s,2H),6.87 (d,2H),7.31 (m,3H). 7.52 (d,2H),7.76 (m,4H),8.03 (d,2H),8.65 (d, 4H),8.76 (d,2H); TPYOHSnPh2Cl2: 3.52 (m,4H),3.61 (m,4H),4.87 (s,2H),6.87 (d,2H),7.32 (m,4H),7.43 (m,4H),7.52 (d,2H),7.78 (d, 4H),7.93 (d,2H),8.03 (d,2H),8.65 (d,4H),8.76 (d,2H). ESI-MS(+p) m/z: 716.1,617.1,596.7,721.5 and 679.5 could be assigned as ANTPYSnMeCl2+,TPYOHSnMeCl2+,TPYOHSnMe2Cl+,TPYOHSnPh2Cl+, and TPYOHSnPhCl2+,respectively.
When ANTPYwas treated with the four organotin(IV) precursors, insoluble solid were obtained except for MeSnCl3,which could form a deep red coordination complex with acceptable solubility in DMSO. Crystals suitable for X-ray diffraction were obtained by diffusion of acetone into the DMSO solution. However,TPYOH could react with all the four organotin(IV) precursors to obtain related Sn(IV) complexes with good solubility in common organic solvents.
Deduced from the ESI-MS data,1H NMR spectra and the singlecrystal X-ray diffraction results,all five mononuclear Sn(IV) complexes were six-coordinated. The molecular structure and atom labeling of the ligands and tin complexes were shown in Fig. 2. The tin complex of ANTPY had a distorted octahedral coordination geometry and centralmetalwas connected by the {N,N,N} atoms in ANTPY together with two chloride atoms and a methyl group,and the leaving Cl atomserved as the counteranion. The bond lengths of the Sn-Cl,Sn-C and Sn-N were in accordance with the literature reports [3, 4, 5].
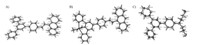
|
Download:
|
| Fig. 2.ORTEP drawing of the terpyridine ligands and tin complex (a) ANTPY,(b) ANTPYSnMeCl3 and (c) TPYOH. Ellipsoids are drawn at 30% probability. All the solvent molecules and the counteranion were omitted for clarity. Detailed crystallographic data can be obtained free of charge from the Cambridge Crystallographic Data Center with the CCDC reference numbers of 839106,959443,855404. | |
As can be seen from Fig. 3,the TPYOH displayed a evident fluorescent emission at 460 nm and the emission band of the four Sn(IV) complexes showed notable bathochromic shifts (3-13 nm) when compared with the free ligand. All four emission bands showed a potent hypochromism. The fluorescent intensity of the Sn(IV) complexes with two coordinated methyl/phenyl groups decreased by 41%/62%,while only a 16% and 36% decrease in intensity were found for the complexes with one phenyl or methyl group. Similarly,the ANTPY ligand exhibited a strong emission at 487 nm,while its Sn(IV) complex showed a 8 nm bathochromic shift peak with a 35% decrease in intensity.

|
Download:
|
| Fig. 3.Normalized fluorescence emission of the terpyridine ligands and their Sn(IV) complexes in CH3CN. The concentration was 1.0 × 10-5 mol/L. | |
The cytotoxicity of five Sn(IV) complexes against two human carcinoma cell lines was measured by the MTT assay,and the IC50 values determined from the dose-dependence of the surviving cells after exposure to the complexes for 48 h are listed in Table 1. All the tin complexes showed significantly higher cytotoxicity against human cancer cells than cisplatin and the free terpyridine ligands. The preliminary results showed that the complexes with two coordinated Cl atoms were more cytotoxic than those with only one.
| Table 1 IC50 (mmol/L) data for the tin-terpyridine complexes and ligands. All values were calculated using the data from three replicate tests. |
Five mononuclear organotin complexes of terpyridine were synthesized and characterized. They demonstrated strong fluorescent properties and higher cytotoxicity than cisplatin against two tumor cell lines tested. Further studies are required to outline a clearer structure-activity relationship. The antitumor mechanism of the tin(IV)-terpyridine remains to be clarified. Nevertheless, the current results form an important starting point for future development of tin-terpyridine complexes with therapeutic potentials.
We are grateful for the financial supports from the Natural Science Foundation of China (No. 21101069). Dr. Shi also thanks the Jiangsu Overseas Research& Training Program for University Prominent Young & Middle-aged Teachers and Presidents.
| [1] | N. Mala, K.S. Pramendra, Chemistry and applications of organotin(Ⅳ) complexes of Schiff bases, Dalton Trans. 40 (2011) 7077-7121. |
| [2] | U.S. Schubert, A. Winter, G.R. Newkome, Optoelectronic and Life Science Applications, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2011. |
| [3] | D.V. Naik, W.R. Scheidt, Crystal and molecular structure of dimethyldiisothiocyanato( terpyridyl)tin (Ⅳ), Inorg. Chem. 12 (1973) 272-276. |
| [4] | F.W.B. Einstein, B.R. Penfold, The crystal structure of a compound containing tin(Ⅳ) inboth5-fold and6-fold co-ordination:[(CH3)2SnCl,terpyridyl]+[(CH3)2SnCl3] , J. Chem. Soc. A (1968) 3019-3024. |
| [5] | E. Najafi, M.M. Amini, S.W. Ng, Dimethyl(4'-pyridyl-2,2':6',2''-terpyridine k3N1N1', N1'')bis(thiocyanato-kN)-tin(Ⅳ), Acta Cryst. Sec. E 67 (2011) m211. |
| [6] | N.W.G. Debye, E. Rosenberg, J.J. Zuckerman, Tin-119m Mössbauer study of fiveand six-coordinated organotin(Ⅳ) ions, J. Am. Chem. Soc. 90 (1968) 3234-3236. |
| [7] | S.H. Li, F.R. Chen, Y.F. Zhou, et al., Enhanced fluorescence sensing of hydroxylated organotins by a boronic acid-linked Schiff base, Chem. Commun. (2009) 4179- 4181. |
| [8] | O. Johansson, M. Borgstro¨m, R. Lomoth, et al., Electron donor-acceptor dyads based on ruthenium(Ⅱ) bipyridine and terpyridine complexes bound to naphthalenediimide, Inorg. Chem. 42 (2003) 2908-2918. |
| [9] | P.F. Shi, Q. Jiang, Preparation of water-soluble terpyridyl fluorescent compound, CN 102584686 A. |
| [10] | M.X. Lin, X.Y. Wang, J.H. Zhu, et al., Cellular and biomolecular responses of human ovarian cancer cells to cytostatic dinuclear platinum(Ⅱ) complexes, Apoptosis 16 (2011) 288-300. |





