b Department of Energy and Chemical Technology,Ningxia Polytechnic & TV University,Yinchuan 750021,China
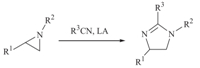
The preparation of imidazolines analogs has attracted consid- erableinterest[1]duetotheirpotentialbiologicalactivities[2]and applications in organocatalysis [3]. The Ritter reaction provides an important route to generate amides via stabilized carbocations. Originally,the carbocations employed are generated from alkenes [4] and alcohols [5] in the presence of Brønsted acids. Recently, epoxides [6] and aziridines [7],as available carbonium ion sources, have been employed and proven successful in the Ritter process. Utilizing the Ritter reaction,the products from epoxides and aziridines are dihydrooxazoles and imidazolines,respectively, followed with ring closure (Scheme 1). Although several groups have reported that the combination of nitriles and aziridines with the promotion of a Lewis acid can give imidazolines [7],however, the deficiencies of the reported procedures,including expensive reagents[7e, 7f],hightemperature[7a, 7d, 7e]andunsatisfiedyields [7a, 7b, 7d, 7g],tended to limit their applications. The reported yields are generally from 60% to 80% and some use lanthanide triflates [7e] as the promoter for this conversion. To our surprise, with aziridines and epoxides only Lewis acids have been reported to be successful,and the most widely referenced one is BF3?OEt2. The Lewis acids employed in the process are (frequently) moisture sensitive,and moreover,some Lewis acids result in undesired products,since the halide anion from the promoter can act as a nucleophile to attack the aziridine ring,or the intermediate. For example in reference [7f],InCl3,ErCl3and YbCl3do not give any Ritter products,but deliver chlorinated compounds as the major product,while Et3OBF4results in some fluorinated product [7b] accompanied with the imidazoline. Furthermore,the imidazolines could be easily hydrolyzed to diamine derivatives with the promotion of HCl in EtOH in very high yield [8]. Considering the important application of diamines,the efficient preparation of imidazolines is of potential and high demand. Herein,we describe a practical preparation of imidazolines in excellent yields from aziridines and nitriles at room temperature.
|
Download:
|
| Scheme 1. Representative Ritter process of nitrile and aziridine. | |
As mentioned above,we focused our attention on the use of Brønsted acids for the Ritter transformation. Aziridine 1a and acetonitrile 2a were selected for the model reaction because of their frequent appearance in other reported experiments. Initially, we applied 1 equiv. of Brønsted acid as the promoter (Table 1, entries 1-6),while BF3?OEt2was used as comparison. Among the Brønsted acids,H2SO4,aqueous HCl,TFA,HClO4 and trifluoro- methanesulfonic acid (TfOH) were screened,but TfOH was demonstrated the most efficient and gave the corresponding imidazoline 3a in 72% yield,as the control subject,and in contrast, BF3?OEt2delivered 3a in 60% yield.
| Table 1 Screening of acids for reaction of 1a and acetonirile.a |
After TfOH was identified as the promoter,we switched our attention to the nitriles,since acetonitrile was widely used in the literature. Apart from it,aliphatic nitriles,including propiononi- trile and isobutyronitrile,were expanded,and gave the corre- sponding imidazolines in similar yields to that of acetonitrile [7b, 7g]. There are a variable number of protons at the positiona to the nitrile group,and we reasoned that these protons could probably affect the process. To the best of our knowledge, pivalonitrile 2b was not reported for this transformation. We conducted the experiment with pivalonitrile 2b and aziridine 1a in the presence of 1 equiv. of TfOH for 24 h and obtained the imidazoline 3b in 84% yield (Table 1,entry 7). This indicated that nitrile 2b would be better than acetonitrile 2a (Table 1,entry 7 vs. entry 6). In order to achieve a higher yield,we increased TfOH from 1 equiv. to 1.2 equiv. and 1.5 equiv.,the yield of 3b was improved to 92% and 95%,while the reaction time remained at 24 h (Table 1, entry 8 and entry 9). Then 2.0 equiv. of TfOH was attempted,3b was obtained in 96% yield,and the reaction time was shortened to 6 h (Table 1,entry 10). The acetonitrile 2a was also reacted with aziridine 1a under the above conditions,and 3a was generated in 89% yield (Table 1,entry 11). The reaction conditions were optimized as follows: Treatment of 1 equiv. of the aziridine with 2.0 equiv. of TfOH in nitrile at room temperature for the required time. Furthermore,the imidazoline 3a could be obtained in 95% yield in 0.7 h by slightly increasing the amount of the promoter to 2.5 equiv. (Table 1,entry 12),which was the best yield with the same substrates in the reports,and the common yield was only around 70% [7].
A represent procedure for preparation of imidazoline 3: To a stirred solution of 1a (272 mg,1.0 mmol) in pivalonitrile 2b (2 mL) was added TfOH (300 mg,2.0 mmol). After stirring at room temperature for an additional 6 h,the mixture was concentrated andtheresiduewaschromatographedtoaffordimidazoline3basa yellow oil (342 mg,96% yield from 1a):1H NMR (400 MHz,CDCl3): d 1.56 (s,9 H),2.42 (s,3 H),3.59 (dd,1H,J = 8.2 Hz and 10.8 Hz), 4.13 (dd,1H,J = 9.6 Hz and 10.8 Hz),4.78 (t,1H,J = 9.2 Hz),7.06- 7.08 (m,2 H),7.24-7.29 (m,5 H),7.74 (d,2H,J = 8.0 Hz);13C NMR (100 MHz,CDCl3):d 21.6,29.4 (3),36.7,58.4,66.3,126.4 (2),127.2 (2),127.4,128.6 (2),129.9 (2),136.5,141.5,144.3,167.7; IR (film, cm?1): 2962,1617,1351,1169,1003,668; MS (EI): m/z 341,291, 200,173,117,98,91; HRMS (ESI) calcd. for C20H25N2O2S (M++H): 357.1631,found: 357.1625.
The analytical data for all the other products and copies of 1HNMRand13CNMRspectracanbefoundinSupportinginformation.
3. Results and discussionTo further demonstrate the efficiency of the reaction,a series of substrates were investigated (Table 2). Besides the aliphatic nitriles,the aromatic nitrile PhCN 2c was examined (Table 2,entry 1),it proceeded well with aziridine 1a and gave the imidazoline 3c in 80% yield. Increasing the hindrance by introducing a methyl group to the o-position of aromatic ring in the nitrile did not decreasetheyield(Table2,entry2),theyieldofthetransformation remained at 80% but required a longer time (12 h vs. 6 h). Aliphatic substituted aziridine 1e was also reacted with 2b and gave the imidazoline 3e in 90% yield (Table 2,entry 3). It should be noted that the regioselectivity was altered mainly due to the reaction proceeding via SN2 mode where the attack took place at the less hindered site. The structure of imidazoline 3e was assigned by comparison to the similar compound reported [7g]. Cycloalkyl aziridines 1f and 1g could also be successfully transformed to the bicyclicimidazolinesinhighyields,butwewereunabletoseparate them from the reaction mixtures. After column chromatographic purification,the hydrolyzed products 3f and 3g were obtained in 86% and 85% yield (Table 2,entries 4 and 5).
| Table 2 TfOH promoted Ritter reaction of alkylaziridines and nitriles.a |
In conclusion,we have developed an efficient procedure for the preparation of imidazolines from aziridines and nitriles. The procedure employed the Brønsted acid TfOH as the promoter, and it indicated that pivalonitrile was better than acetonitrile. The imidazoline could be obtained in 80%-96% yield. Furthermore, even the acetonitrile could deliver the imidazoline in excellent yield by slightly increasing the amount of TfOH. The reaction was performed at room temperature and no expensive promoters were used,and the yields were much better than those of the reported procedures.
AcknowledgmentsThis work was supported by the National Natural Science FoundationofChina(Nos.21262024,21062014),theKey Projectof Chinese Ministry of Education (No. 211193),the Scientific Research Foundation for Returned Scholars (Ministry of Education of China),the Natural Science Foundation of Ningxia (No. NZ1165), the Key Project of Department of Education in Ningxia (2010- Preparation of Capsaicin),the 100 Talents Program of Ningxia, and the ‘‘211’’ Project in Ningxia University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.020.
| [1] | R.D. Crouch, Synthetic routes toward 2-substituted 2-imidazolines, Tetrahedron 65 (2009) 2387-2397. |
| [2] | (a) G.W. Bemis, M.A. Murcko, The properties of known drugs. 1. Molecular frameworks, J. Med. Chem. 39 (1996) 2887-2893; (b) P. Janvier, X.W. Sun, H. Bienayme, J.P. Zhu, Ammonium chloride-promoted four-component synthesis of pyrrolo[3,4-b]pyridin-5-one, J. Am. Chem. Soc. 124 (2002) 2560-2567. |
| [3] | (a) P.I. Dalko, Y. Langlois, Stereoselective preparation of quaternary benzylic centres using chiral imidazolines, Chem. Commun. (1998) 331-332; (b) T. Morimoto, K. Tachibana, K. Achiwa, ChemInform abstract: asymmetric reactions catalyzed by chiral metal complexes. Part 78. Chiral thioimidazoline ligands for palladium-catalyzed asymmetric allylation, Synlett 28 (1997) 783-785. |
| [4] | (a) J.J. Ritter, P.P. Minieri, A new reaction of nitriles. I. Amides from alkenes and mononitriles, J. Am. Chem. Soc. 70 (1948) 4045-4048; (b) J.J. Ritter, J. Kalish, A new reaction of nitriles. Ⅱ. Synthesis of t-carbinamines, J. Am. Chem. Soc. 70 (1948) 4048-4050. |
| [5] | (a) M.Y. Lebedev, M.B. Erman, Lower primary alkanols and their esters in a Rittertype reaction with nitriles. An efficient method for obtaining N-primary-alkyl amides, Tetrahedron Lett. 43 (2002) 1397-1399; (b) M. Dos Santos, B. Crousse, D. Bonnet-Delpon, Improved Ritter reaction with CF3-containing oxirane for an access to central units of protease inhibitors, Tetrahedron Lett. 50 (2009) 857-859; (c) P. Rubenbauer, T. Bach, Diastereoselective Ritter reactions of chiral secondary benzylic alcohols, Chem. Commun. (2009) 2130-2132. |
| [6] | R.M.A. Pinto, J.A.R. Salvador, C. Le Roux, Ritter reaction mediated by bismuth(ⅡI) salts: one-step conversion of epoxides into vic-acylamino-hydroxy compounds, Synlett (2006) 2047-2050. |
| [7] | (a) T. Hiyama, H. Koide, S. Fujita, H. Nozaki, Reaction of N-alkoxycarbonylaziridines with nitriles, Tetrahedron 29 (1973) 3137-3139; (b) B.A.B.Prasad,G.Pandey,V.K.Singh,Synthesisofsubstitutedimidazolinesvia[3 + 2]- cycloaddition of aziridineswith nitriles, Tetrahedron Lett. 45 (2004) 1137-1141; (c) V.K. Yadav, V. Sriramurthy, Silylmethyl-substituted aziridine and azetidine as masked 1,3- and 1,4-dipoles for formal [3 + 2] and [4 + 2] cycloaddition reactions, J. Am. Chem. Soc. 127 (2005) 16366-16367; (d) M.K.Ghorai,K.Das,A.Kumar,K.Ghosh,Anefficientroutetoregioselectiveopeningof N-tosylaziridineswith zinc(Ⅱ) halides, Tetrahedron Lett. 46 (2005) 4103-4106; (e) J.Wu,X.Y.Sun,H.G.Xia,Sc(OTf)3-catalyzed[3 + 2]-cycloadditionofaziridineswith nitriles under solvent-free conditions, Tetrahedron Lett. 47 (2006) 1509-1512; (f) M.K.Ghorai,K.Ghosh,K.Das,Copper(Ⅱ)triflatepromotedcycloadditionofa-alkylor aryl substituted N-tosylaziridines with nitriles: a highly efficient synthesis of substituted imidazolines, Tetrahedron Lett. 47 (2006) 5399-5403; (g) S.Gandhi,A.Bisai,B.A.B.Prasad,V.K.Singh,Studiesonthereactionofaziridineswith nitriles and carbonyls: synthesis of imidazolines and oxazolidines, J. Org. Chem. 72 (2007) 2133-2142. |
| [8] | K.I. Booker-Milburn, D.J. Guly, B. Cox, P.A. Procopiou, Ritter-type reactions of Nchlorosaccharin: a method for the electrophilic diamination of alkenes, Org. Lett. 5 (2003) 3313-3315. |






