b Department of Chemistry, South China University of Technology, Guangzhou 510641, China;
c Department of Chemistry, Michigan State University, E. Lansing, MI 48824, USA
Corrole is a ring-contracted porphyrin analog having one direct pyrrole-pyrrole link [1]. It represents a distinctive family of macrocyclic compounds with unique structural,chemical,and photophysical properties [2, 3, 4, 5]. Unlike porphyrin [6],corrole contains three protons in the inner core of the ring which renders it a tri-anionic ligand capable of stabilizing metals in high oxidation states [7]. This feature is mainly responsible for metallocorroles acting as potent catalysts in a variety of chemical reactions,such as epoxidation,cyclopropanation,asymmetric sulfoxidation,aziridination, and oxygen atom transfer reactions [8].
Manganese complexes of corroles and related macrocycles are typically employed in various oxygenation reactions to get insights into the reactionmechanismowing to their efficiency and specificity [8, 9, 10, 11, 12]. Manganese corroles are found to be effective catalysts for selective epoxidation of olefins at less-substituted double bond [13], as well as selective oxidation of unactivated C-H bonds in alkanes [14]. It has recently been reported that mild and roomtemperature oxidation of electron rich alcohols to carbonyl compounds may be achieved through manganese corroles bearing electronegative substituents [15]. Significantly less attention,however,has been paid to the catalytic effects of axial ligation in metallomacrocycles. The catalytic activity and selectivity ofmetalloporhyrins and related transition metal complexes in biomimetic oxidation of cytochrome P-450 is substantially affected by the fifth coordination [16]. The Ndonor axial ligands are the most common ones used in the metalloporphyrin catalyzed oxidation systems. Importantly,the rate of oxidation reactions catalyzed by metalloporphyrins could be strongly enhanced bythe additionof an axial ligand,suchaspyridine or imidazole bases [17, 18]. For asymmetric epoxidation,addition of axial ligands to catalytic reaction systems is a convenient and efficient way to enhance enantioselectivity [19, 20]. Metalloporphyrins with covalently linked axial ligand ‘‘tails’’ are often used in the biomimetic modeling [21]. The use of a covalently linked axial ligand may avoid the problem of exogenous ligand oxidation competing with the substrate oxidation. Despite the significant effect of axial ligation in metalloporphyrin catalyzed systems, similar investigations on metallcorroles are scarce,and to the best of our knowledge,only one such report is found in the literature [22] and,in addition,no report exists for the catalysis by ligandappendedmetallocorroles. Herein,wewish to report the synthesis of manganese corroles with covalently attached nitrogenous ligands and their catalytic activities. The catalytic epoxidation of alkenes revealed that the presence of appended N-donor ligands will significantly enhance the rate of OAT from (oxo)manganese(V) corroles to alkenes.
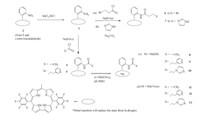
The appended corroles were prepared as shown in Scheme 1 starting from 5-(pentafluorophenyl)-dipyrromethane 1 [23]. The trans-A2Bcorroles 2 and 3 were prepared by our previously reported procedure [24]. The synthetic detail,including synthesis,characterization and catalysis data,are given in Supporting information.
All synthesized appended corroles and their manganese complexes were characterized by spectroscopic methods. The Soretband of appendedmanganese corroles 8,9 and 10 appeared at about 400 nmand 420 nm(Fig. S1 in Supporting information),while theQband of 9,10 exhibited a significant red shift due to the coordination between the tailed,appended ligand and the manganese. The most definitive difference upon appended ligand coordination is the dramatic increase in the absorbance at about 490 nm.
|
Download:
|
| Scheme 1.Synthesis of appended free base corroles and their manganese complexes. | |
Axial ligation exerts a profound effect on the physicochemical properties of metallocorroles similar to metalloporphyrins [19, 20]. The spectral changes in manganese(III) corrole 8 on addition of Nmethylimidazole is shown in Fig. S2 in Supporting information. Excellent isosbestic points were observed,indicating a clean transformation of 8 to its axial coordinated product. Quantitative analysis of the spectroscopic titration data revealed that 8 formed a 1:1 complex with N-methylimidazole,and the binding constant was found to be 1.87 × 104 L/mol. The complexes 9 and 10 also formed a 1:1 complex with N-methylimidazole,but their binding constants were two orders of magnitude less than that of 8. This is due to the coordination between the appended nitrogenous ligand and the core metal in 9 and 10. The binding constants between Mn(III) corrole and N-methylimidazole follows the order Kb8 > Kb9 > Kb10 (Table S1 in Supporting information). The binding constant for pyridine and 8 is 19.4 L/mol,which is comparable to the binding between pyridine and corrole (OMC)Mn [25] (where OMC = octamethylcorrole). The binding between pyridine and 9,10 was too weak to be measured reliably. The binding constant for triethylamine and 8,9 was determined to be 1.08 × 102 L/mol and 2.20 L/mol,respectively,and the binding between triethylamine and 10 was too weak to be measured. It is obvious that these appended manganese(III) corroles exhibit good binding tendency with N-methylimidazole among the examined N-donor ligands,and the binding ability of triethylamine is stronger than pyridine. Manganese(III) corroles are generally fourcoordinated, as in the case of the complex 8,where the coordination of acetamido-ligand is not possible with the manganese center. However,complex 8 will turn to fivecoordinated state in the presence of the external axial ligand. The presence of the appended ligand in complexes 9 and 10 will increase the coordination number from four to five,and only one nitrogenous ligand can coordinate with Mn(III) to form a maximum of five-coordinated complex.
|
Download:
|
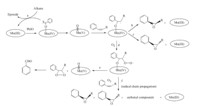
| Scheme 2.Proposed mechanism for the catalytic oxidation of alkenes by appended Mn(III) corroles. | |
High valent (oxo)manganese(V) corroles are the active oxidants in the catalytic cycle of manganese corroles-catalyzed oxidation reactions of alkenes [13, 26] and sulfides [27]. In order to investigate the effects of a tailed axial ligand on the reactivity of the (oxo)manganese(V) corroles,we prepared (oxo)manganese(V) corroles 11,12 and 13 by using PhIO as oxidant [26] and performed kinetic studies on their reactions with styrenes. The UV-vis spectra of these (oxo)manganese(V) corroles resemble those found in literatures [8, 27]. At room temperature,these metal-oxo species decompose gradually and eventually return to the corresponding manganese(III) corroles as indicated by UV-vis data. Addition of styrene can significantly speed up the reduction of these (oxo)manganese corroles. The UV-vis changes of 11 upon mixing with styrene in CH2Cl2 is shown in Fig. S3 in Supporting information. With excess styrene,the reactions followed pseudofirst order kinetics. The rate constants (kobs) and the pseudo-first order reaction activation energy (EA) of the OAT can be derived according to the rate law and Arrhenius equation. The rate constants and activation energy data are summarized in Table S2 in Supporting information. The results reveal that manganese corroles with appended pyridine and imidazole ligands can enhance the OAT process. The half-lives of the acetamido-,pyridyland imidazolyl-appended (oxo)manganese(V) corroles 11,12 and 13 in the presence of styrene (7.28 × 10-2 mol/L) are 150 min, 80 min and 7 min at 25 8C and the activation energies of the pseudo-first order reaction is 27.8 kcal/mol,21.8 kcal/mol and 14.3 kcal/mol,respectively,which indicate that N-donor type axial ligands will provide enhancement on the rate of OAT by lowering the activation energy. The OAT rate follows the order: kobs13 > kobs12 > kobs11. By comparing the rate and the activation energy between the pyridyl-appended and the imidazolylappended (oxo)manganese(V) corroles (12,13),it is evident that a stronger binding may contribute significant enhancement on the rate of an oxidation reaction. The binding of manganese(III) corroles with imidazole is found to be stronger than with pyridine (Table S1). The coordination of nitrogenous ligand at the position trans to the manganese-oxo bond could induce the elongation of the Mn-O bond distance and leads to destabilization of the high valent (oxo)manganese(V) intermediate and thus increases the rate of reduction of (oxo)manganese corroles [22, 28].
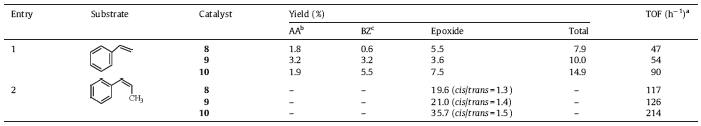
| Table 1 Catalytic epoxidation of alkenes by appended manganese(III) corroles in the presence of PhIO. |
The catalytic oxidation data of styrene and cis-b-methylstyrene by appended Mn(III) corroles is summarized in Table 1. Epoxides were the major products in all cases as indicated by GC analysis. Catalytic oxidation produced styrene epoxide,arylacetylaldehyde and benzaldehyde. The turnover frequency (TOF) for the catalytic oxidation decreased in the order 10 > 9 > 8. The pyridyl appended Mn(III) corrole 9 exhibited only asmall improvement in the catalytic activity as comparedwith acetyl appendedMn(III) corrole8,whilein the case of imidazole appended Mn(III) corrole 10,a significant increase in the yields of total oxidation products was observed. The productionof trans-epoxide fromcis-b-methylstyrene indicates that the intermediatewhichleadsto the direct formationof epoxidemust undergo a cis/trans inversion,and this suggests that themechanism may involve radical intermediates,as observed in manganese porphyrin catalyzed oxidation of alkenes [29]. The proposed mechanism for catalytic oxidation of alkenes by Mn(III) corroles is depicted in ScheMe2.Collmanet al. [30]performedaMn(III) corrolecatalyzed competitive epoxidation of styrene and cyclooctene and demonstrated that there is at least one other active intermediate in addition to (oxo)manganese(V) corrole,and suggested that PhIOMn( IV)corrole adductmay also be an active intermediate (ScheMe2, path a). Recent studies on manganese porphyrins and related macrocyclic systems also demonstrate the existence of multiple active oxidants in the oxygenation reactions [31].
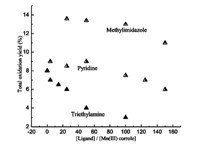
The effect of external axial ligands on appended acetamido manganese corrole 8 catalyzed epoxidation of styrene is shown in Fig. 1. Pyridine,triethylamine and N-methylimidazole were used as axial ligands. In case of triethylamine,as axial ligand,which uses sp3-N for donating electrons,the yield of total oxidation products was observed to decrease sharply with increasing ligand concentration. For pyridine and N-methylimidazole,however,the yield was increased with the increasing concentration of up to [ligand]/ [Mn(III) corrole] < ~50. Further increase in the ligand concentration caused a drop in the total oxidation yield,which may be due to the more effective oxidation of the axial ligand leading to a decrease in the turnover rate. This indicates that N-oxidation of the nitrogenous ligand becomes important at higher concentration. Moreover,the addition of N-methylimidazole may induce a greater improvement on the yields of total oxidation products than pyridine,which may be attributed to the relatively less steric effect and more trans-effect of N-methylimidazole than pyridine.
|
Download:
|
| Fig. 1.Effect of external axial ligands on appended acetamido manganese(III) corrole 8 catalyzed epoxidation of styrene. | |
In summary,wereportthesynthesisandthefirstcatalyticactivity of N-base appended manganese corrole complexes. The appended manganese(III) corroles normally form1:1 complexes with N-donor axial ligands,and give enhancement on the rate of oxygen atom transfer from the corresponding (oxo)manganese(V) corrole to the substrate. The advantage of ligand appendedmanganese(III) corrole in the catalytic oxidation of substrate is to provide a donor ligand without the drawback of competitive oxidation of the excess ligand, and demonstrates the convenience of using easily available organic bases,suchaspyridineandN-methylimidazole,toenhancetheyields of catalytic oxidation products.
This work was supported by National Natural Science Foundation of China (Nos. 21171057,21371059). Research Grant Council of Hong Kong under project HKUST6182/99p and Area of Excellence Scheme (No. AoE/P-10/01).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.027.
| [1] | R. Paolesse, Corrole: the little big porphyrinoid, Synlett 15 (2008) 2215-2230. |
| [2] | W.L. Shao, H. Wang, S. He, et al., Photophysical properties and singlet oxygen generation of three sets of halogenated corroles, J. Phys. Chem. B 116 (2012) 14228-14234. |
| [3] | L. Shi, H.Y. Liu, L.P. Si, et al., The heavy atom effect on photocleavage of DNA by mono-hydroxyl halogentaed corroles, Chin. Chem. Lett. 21 (2010) 373-375. |
| [4] | P. Lian, H.Y. Liu, L.Y. Liu, et al., Synthesis of binaphthyl bridged chiral bis-corrole, Chin. Chem. Lett. 20 (2009) 21-24. |
| [5] | H.Y. Liu, L. Chen, F. Yam, et al., Reductive demetalation of manganese corroles: the substituent effect, Chin. Chem. Lett. 19 (2008) 1000-1003. |
| [6] | M.H.R. Mahmood, H.Y. Liu, H.H. Wang, Y.Y. Jiang, C.K. Chang, Unexpected one-pot synthesis of A3-type unsymmetrical porphyrin, Tetrahedron Lett. 54 (2013) 5853-5856. |
| [7] | A.E. Meier-Callahan, H.B. Gray, Z. Gross, Stabilization of high-valent metals by corroles: oxo[tris(pentafluorophenyl)corrolato]chromium(Ⅴ), Inorg. Chem. 39 (2000) 3605-3607. |
| [8] | H.Y. Liu, M.H.R. Mahmood, S.X. Qiu, C.K. Chang, Recent developments in manganese corrole chemistry, Coord. Chem. Rev. 257 (2013) 1306-1333. |
| [9] | J. Lu, H.Y. Liu, L. Shi, et al., DNA cleavage mediated by water-soluble manganese corrole, Chin. Chem. Lett. 22 (2011) 101-104. |
| [10] | L. Yu, Q. Wang, L. Dai, et al., Solvent effects on oxygen atom transfer reaction between manganese(Ⅴ)-oxo corrole and alkene, Chin. Chem. Lett. 24 (2013) 447- 449. |
| [11] | Y. Zhang, Q. Wang, J.Y. Wen, et al., DNA binding and oxidative cleavage by a water-soluble carboxyl manganese(ⅡI) corrole, Chin. J. Chem. 31 (2013) 1321- 1328. |
| [12] | J.T. Huang, X.L. Wang, Y. Zhang, et al., DNA binding and nuclease activity of a water-soluble sulfonated manganese(ⅡI) corrole, Transit. Met. Chem. 38 (2013) 283-289. |
| [13] | H.Y. Liu, F. Yam, Y.T. Xie, X.Y. Li, C.K. Chang, A bulky bis-pocket manganese(Ⅴ)-oxo corrole complex: observation of oxygen atom transfer between triply bonded MnVBO and alkene, J. Am. Chem. Soc. 131 (2009) 12890-12891. |
| [14] | S. Bose, A. Pariyar, A.N. Biswas, et al., Electron-deficient manganese(ⅡI) corrole catalyzed oxidation of alkanes and alkylbenzenes at room temperature, Catal. Commun. 12 (2011) 1193-1197. |
| [15] | S. Bose, A. Pariyar, A.N. Biswas, et al., Manganese(ⅡI) corrole catalyzed selective oxidation of alcohols to carbonyl compounds by tert-butyl hydroperoxide under mild conditions, Catal. Commun. 12 (2011) 446-449. |
| [16] | K. Jitsukawa, H. Shiozaki, H. Masuda, Epoxidation activities of mononuclear ruthenium-oxo complexes with a square planar 6,60-(benzoylamino)-2,20-bipyridine and axial ligands, Tetrahedron Lett. 43 (2002) 1491-1494. |
| [17] | J.P. Collman, J.I. Brauman, P.D. Hampton, et al., Mechanistic studies of olefin epoxidation by a manganese porphyrin and hypochlorite: an alternative explanation of saturation kinetics, J. Am. Chem. Soc. 112 (1990) 7980-7984. |
| [18] | J.T. Groves, M.K. Stern, Olefin epoxidation by manganese(Ⅳ) porphyrins: evidence for two reaction pathways, J. Am. Chem. Soc. 109 (1987) 3812-3814. |
| [19] | T.S. Lai, K.H. Ng, H.Y. Liu, C.K. Chang, L.L. Yeung, Effect of fifth coordination in catalytic epoxidation by a chiral manganese porphyrin, Synlett 9 (2002) 1475- 1478. |
| [20] | T.S. Lai, S.K.S. Lee, L.L. Yeung, et al., Remarkable axial ligand effect on regioselectivity towards terminal alkenes in epoxidation of dienes by a robust manganese porphyrin, Chem. Commun. (2003) 620-621. |
| [21] | J.P. Collman, R. Schwenninger, M. Rapta, M. Broring, L. Fu, New 1,4,7-triazacyclononane- based functional analogues of the Fe/Cu active site of cytochrome c oxidase: structure, spectroscopy and electrocatalytic reduction of oxygen, Chem. Commun. (1999) 137-138. |
| [22] | H.Y. Liu, H. Zhou, L.Y. Liu, et al., The effect of axial ligand on the reactivity of oxomanganese(Ⅴ) corrole, Chem. Lett. 36 (2007) 274-275. |
| [23] | C.H. Lee, J.S. Lindsey, One-flask synthesis of meso-substituted dipyrromethanes and their application in the synthesis of trans-substituted porphyrin building blocks, Tetrahedron 50 (1994) 11427-11440. |
| [24] | X. Ying, X.Y. Long, M.H.R. Mahmood, et al., Second order nonlinear optical properties of corroles: experimental and theoretical investigations, J. Porphyrins Phthalocyanines 16 (2012) 1276-1284. |
| [25] | S. Licoccia, E. Morgante, R. Paolesse, et al., Tetracoordinated manganese(ⅡI) alkylcorrolates. Spectroscopic studies and the crystal and molecular structure of (7,13-dimethyl-2,3,8,12,17,18-hexaethylcorrolato)manganese(ⅡI), Inorg. Chem. 36 (1997) 1564-1570. |
| [26] | H.Y. Liu, T.S. Lai, L.L. Yeung, C.K. Chang, First synthesis of perfluorinated corrole and its Mn5O complex, Org. Lett. 5 (2003) 617-620. |
| [27] | A. Kumar, I. Goldberg, M. Botoshansky, et al., Oxygen atom transfer reactions from isolated (oxo)manganese(Ⅴ)corroles to sulfides, J. Am. Chem. Soc. 132 (2010) 15233-15245. |
| [28] | K.A. Jørgensen, P. Swanstrøm, On the proximal effect of the nitrogen ligands on the oxomanganese porphyrin systems, Acta Chem. Scand. 43 (1988) 822- 824. |
| [29] | R.D. Arasaingham, G.X. He, T.C. Bruice, Mechanism of manganese porphyrincatalyzed oxidation of alkenes. Role of manganese(Ⅳ)-oxo species, J. Am. Chem. Soc. 115 (1993) 7985-7991. |
| [30] | J.P. Collman, L. Zeng, R.A. Decre′ au, Multiple active oxidants in competitive epoxidations catalyzed by porphyrins and corroles, Chem. Commun. (2003) 2974-2975. |
| [31] | M.Y. Hyun, Y.D. Jo, J.H. Lee, et al., Remarkable solvent, porphyrin ligand, and substrate effects on participation of multiple active oxidants in manganese( ⅡI) porphyrin catalyzed oxidation reactions, Chem. Eur. J. 19 (2013) 1810-1818. |