b Tianjin Key Laboratory of Molecular Design and Drug Discovery, Tianjin Institute of Pharmaceutical Research, Tianjin 300193, China
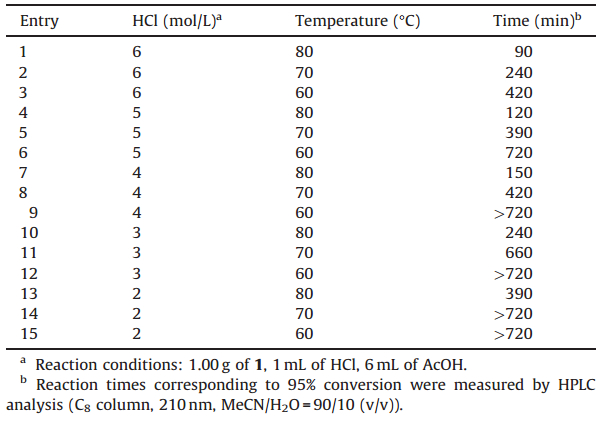
Methyl glycosides constitute an important class of carbohydrate derivatives. Their hydrolysis can produce the corresponding lactols,which are important intermediates for further modifications [1] or can be used to prepare glycosyl donors for the construction of oligosaccharides [2, 3, 4].
Hydrolysis of methyl glycosides to their corresponding lactols is always carried out under rather harsh conditions [5, 6, 7, 8, 9, 10, 11, 12, 13],which are characterized by strongly acidic reaction media and high reaction temperatures [5, 6, 7, 8],and often suffers from poor yields [9, 10, 11, 12] and large quantities of byproducts which can cause great trouble in purification in many cases [12, 13]. Survey of literature revealed that there are no currently available methods to solve these problems. Prompted by these unsolved shortcomings in the current methods,we were interested in finding a cocatalyst to promote the hydrolysis while suppressing the formation of byproducts so that relatively mild hydrolysis conditions could be adopted and the aforementioned drawbacks could be solved. Considering the fact that when methyl glycosides are ready for acidic hydrolysis their hydroxyl groups are usually protected by robust protecting groups,such as alkyl and benzyl groups [5, 6, 7, 8, 9, 10, 11, 12, 13], because most other protecting groups usually cannot survive the harsh hydrolysis conditions,we chose the methyl glycosides with methyl and benzyl protecting groups for our study (Fig. 1). We herein report an efficient cocatalyst,SrCl2,for the acidic hydrolysis of methyl glycosides,which can be highlighted as inexpensive, shortening reaction times,suppressing formation of byproducts and improving yields.
|
Download:
|
| Fig. 1.Substrates used for the SrCl2-cocatalyzed acidic hydrolysis.. | |
The melting points were measured with an XT-4 microscopic melting apparatus and are uncorrected. The 1H NMR spectra were recorded on a Bruker AV400 spectrometer with DMSO-d6 as solvent and TMS as internal standard. HPLC analyses were performed with a Waters 2695 high-performance liquid chromatograph equipped with a Diamonsil C8 column (150 mm X 4.6 mm,5mm; held at 358C) and a UV detector (210 nm), using MeCN/H2O (90/10,v/v) as mobile phase at a flow rate of 0.6 mL/min.
Methyl tetra-O-benzylglycosides1-6were prepared from their corresponding unprotected methyl glycosides following a known one-step procedure [14]. Compounds7and8were prepared from methyla-D-glucopyranoside following known procedures [15, 16], and substrate9was prepared according to a known method [1]. All the substrates1-8are known compounds and their melting points or 1H NMR data were in good agreement with those reported.
Procedure for screening of concentration of HCl and reaction temperature: In a 50 mL round-bottomed flask,1.00 g (1.8 mmol) of1was dissolved in 6 mL of glacial acetic acid,and the solution was stirred and heated to a specific temperature (Table 1). To the mixture was added 1 mL of HCl with a specific concentration (Table 1). The reaction was timed just after the addition of HCl,and aliquots of the reaction mixture were subjected to HPLC analysis at 30 min intervals to follow the reaction course until a conversion of >95% was reached.
|
|
Procedure for screening of the kind of metal salt: In a 50 mL round-bottomed flask,1.00 g (1.8 mmol) of1was dissolved in 6 mL of glacial acetic acid,and the solution was stirred and heated to 708C. To the mixture was added 1 mL of 5 mol/L HCl,followed by addition of 1.0 eq. of metal salt (Table 2). The reaction was timed just after the addition of HCl and metal salt,and aliquots of the reaction mixture were subjected to HPLC analysis at 30 min intervals to follow the reaction course until a conversion of>95% was reached.
|
|
Procedure for screening of equivalent of metal salt: In a 50 mL round-bottomed flask,1.00 g (1.8 mmol) of1was dissolved in 6 mL of glacial acetic acid,and the solution was stirred and heated to 708C. To the mixture was added 1 mL of 5 mol/L HCl,followed by addition of a specific equivalent of metal salt (Table 3). The reaction was timed just after the addition of HCl and metal salt,and aliquots of the reaction mixture were subjected to HPLC analysis at 30 min intervals to follow the reaction course until a conversion of>95% was reached.
|
|
Procedure for acidic hydrolysis of1-9to test the generality of SrCl2as cocatalyst: In a 50 mL round-bottomed flask,1.00 g of substrate1-9was dissolved in 6 mL of glacial acetic acid,and the solution stirred and heated to 708C (Table 4). To the mixture was added 1 mL of 5 mol/L HCl,followed by the addition of 0.10 eq. of SrCl2·6H2O. The reaction was timed just after the addition of HCl and SrCl2·6H2O,and aliquots of reaction mixture subjected to HPLC analysis at 30 min intervals to follow the reaction course until a conversion of>95% was reached. The reaction mixture was quenched by adding 50 mL of ice-water,and the resulting mixture was extracted with CH2Cl2(20 mL Χ 3). The combined extracts were washed with saturated aqueous NaHCO3and brine,dried over anhydrous Na2SO4and evaporated on a rotary evaporator to afford the crude lactols,which were purified by column chromatography to yield the pure lactols10-15. All the lactols 10-15corresponding to1-9are known compounds,and their melting points or 1H NMR were in good agreement with those reported (Table 4).
|
|
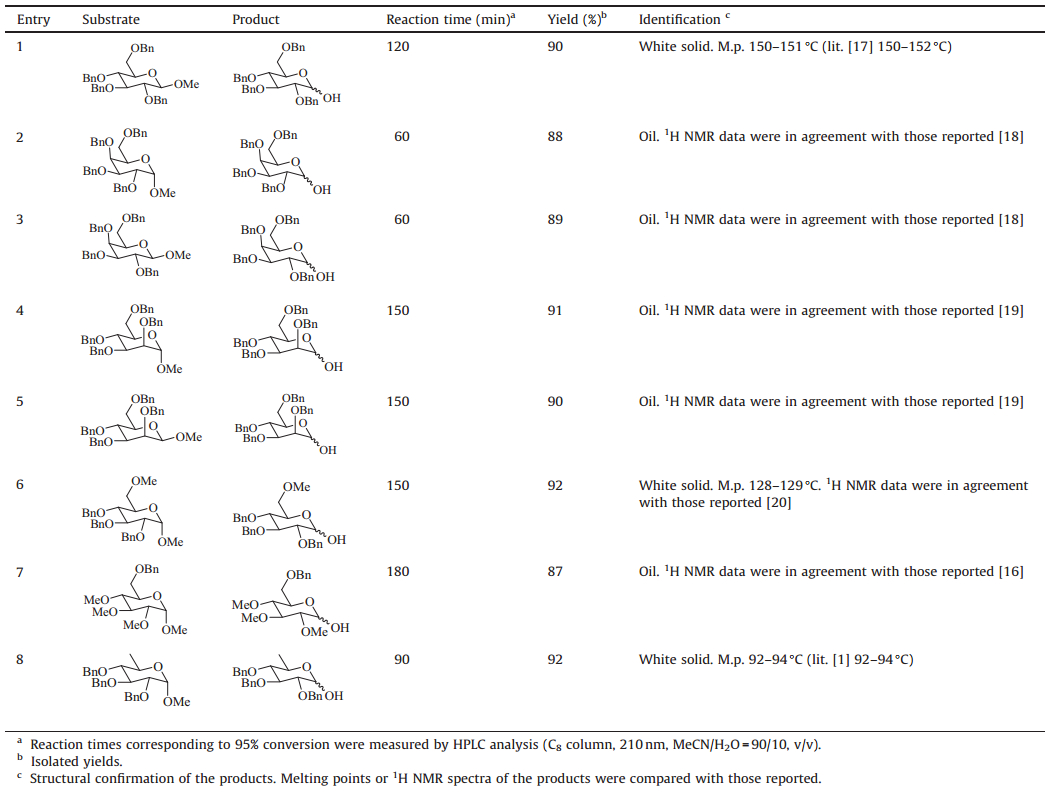
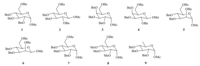
The corresponding lactols produced by hydrolysis of methyl glycosides are versatile intermediates in organic chemistry (Scheme 1). According to our earlier study [1] and the report of Kotoet al. [12],the hydrolysis of methyl glycosides needed rather harsh reaction conditions,most of which involved heating the methyl glycosides in glacial acetic acid at high temperatures with aqueous strong acids,such as H2SO4[10, 11],HCl [7, 8, 12] and TfOH [6]. These harsh reaction conditions could indeed result in quick reactions; however,a main drawback was that the desired product often included large quantities of byproducts,such as debenzylated products [12, 13],complicating the workup and purification procedures and resulting,in many cases,in poor yields [9, 10, 11, 12] due notably to the reaction conditions [12] (Scheme 2). In the normal acidic hydrolysis of methyl glycosides,the protons chelate the glycosidic oxygen atoms aiding the cleavage of the glycosidic bond. Since metal ions are also electron-deficient species which can also chelate these oxygen atoms in an identical way to protons, selection of a metal salt as a Lewis acid cocatalyst to accelerate the hydrolysis step while suppressing the formation of byproducts was desired to solve the problems in the reported methods associated with the hydrolysis of methyl glycosides.
|
Download:
|
| Scheme 1.Examples for hydrolysis of methyl glycosides and the representative applications of lactols thus produced. | |
|
Download:
|
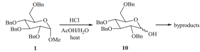
| Scheme 2.Hydrolysis of 1 to its corresponding lactol 10 and the formation of byproducts that follows. | |
With high concentrations of HCl and high temperatures,the acidic hydrolysis of methyl glycosides can proceed quickly,but often leads to large quantities of byproducts and poor yields [12]. So,prior tothe screening of metal salts as cocatalysts,suitable reaction conditions to complete the hydrolysis of methyl glyco sides within a reasonable time should be found because if the hydrolysis rate proceeds too fast or too slow,it will be difficult to clearly detect the effect of the cocatalysts. We chose the commonly encountered methyl 2,3,4,6-tetra-O-benzyl-a-D-glucopyranoside 1(Scheme 2) as the substrate for the initial screening of the two important hydrolysis parameters,i.e.the concentration of the acid and the reaction temperature. It should be noted that HCl,as the acid,and metal chlorides,as the Lewis acid cocatalyst,were used throughout our study because most metal chlorides are soluble in water whereas many of their sulfate counterparts are not. The results are shown in Table 1. The concentration of HCl ranged from 2 mol/L to 6 mol/L,and the reaction temperatures ranged from 608Cto808C. As can be observed clearly in Table 1,the reaction rate responded much more dramatically to the temperature change than to the change of HCl concentration. The reaction rate decreased significantly below 708C and increased quickly above 708C,therefore,708C was selected as the optimum temperature. At 708C,the reaction times ranged from 240 min to>720 min,and finally 390 min was believed to the optimum reaction time at 5 mol/L of HCl and most suitable for further screening of metal salts as cocatalyst (entry 5 in Table 1).
With in hand the optimum conditions for the screening of metal salts,i.e. 5 mol/L HCl at 708C,we proceeded to screen 26 representative metal salts as cocatalyst using 1 as substrate (Table 2). As shown in Table 2,with soft alkali metal ions (entries 1-5) the hydrolysis exhibited a slight improvement compared with that without any metal salt (entry 5 in Table 1); however,they were marked by longer reaction times and moderate yields and impurity profiles. The hard metal ions (entries 10,11,13,17,18 and 26) could considerably accelerate the reaction,but,unfortunately,could lead to the formation of significant levels of byproducts,and dramatically decreased yields. Based on the comprehensive evaluation in terms of reaction time,yield and purity,three metal salts,SrCl2(entry 9),MnCl2(entry 20) and CuCl2 (entry 24),were selected for further investigation and indicated that moderate hardness of the metal ions is best suited for the our purpose. Neither too soft nor too hard metal ions were appropriate for the hydrolysis,with the former ones having insufficient cocatalytic efficiency while the latter ones exhibiting too powerful cocatalytic efficiency that led to decomposition of the products as well.
Since the preliminary screenings used 1.0 eq. of metal salts as shown in Table 2,we determined to study the effect of the quantity of the cocatalyst on the cocatalytic efficiency (Table 3). The reactions were carried out with the three designated metal salts using1as substrate and optimum reaction conditions,i.e.5 mol/L HCl at 708C. As shown in Table 3,as the quantity of cocatalyst increased,the reaction rates with all three cocatalysts gradually increased to reach plateaus. The reaction rate with SrCl2and MnCl2 also displayed slight decreases at specific large quantities (entries 8 and 16). The smallest quantities of these three cocatalysts corresponding to the best reaction rates were then determined as 0.1 eq. for SrCl2,0.8 eq. for MnCl2,and 0.2 eq. for CuCl2. Further examination of the data found that 0.1 eq. of SrCl2was the best because it corresponded to the shortest reaction time (150 min) and highest yield (91%) and impurity profile (95% with 2 impurities). So,SrCl2was finally selected as the best cocatalyst for use at a low concentration of 0.1 eq.
With in hand the best cocatalyst and its quantity (0.1 eq. of SrCl2) under the optimum reaction conditions (5 mol/L HCl at 708C) established,we tested the generality of this cocatalyst with a variety of methyl glycosides as the substrate (Table 4). As shown in Table 4,when substrates 2-9 were subjected to the acidic hydrolysis cocatalyzed by 0.1 eq. of SrCl2and optimizes conditions, the reactions all proceeded very well,completing within short reaction times and furnishing the corresponding lactols in good yields. The results demonstrated that SrCl2 can be used as an efficient cocatalyst for the acidic hydrolysis of methyl glycosides.
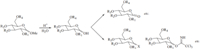
The mechanism for the cocatalysis with SrCl2is postulated as proceedingviathe bidentate chelation of the glycosidic and 2-O atoms to the Sr2+ ion,forming a 5-membered ring which facilitated the acidic hydrolysis that followed (Fig. 2). The 5-membered ring intermediate is consistent to a similar process reported earlier [21]. As can be clearly shown in Fig. 2,the cocatalytic efficiency of a metal ion depends on its hardness: harder metal ions can form more stable 5-membered rings,which can more readily help to cleave the glycosidic bond in the key step of the hydrolysis. This mechanism explains why the hard metal salts (entries 10,11,13, 17,18 and 26 in Table 2) considerably accelerate the hydrolysis, whereas the soft ones (entries 1-5 in Table 2) could not. 4. Conclusion
SrCl2was found to be the most efficient cocatalyst for the acidic hydrolysis of methyl glycosides among 26 metal salts screened as Lewis acid. The SrCl2-cocatalyzed acidic hydrolysis of methyl glycosides was highlighted by short reaction times,less byproducts and high yields. Based on this work,the best conditions for acidic hydrolysis of methyl glycosides included heating the methyl glycosides in a mixed solvent consisting of 5 mol/L HCl/acetic acid (1/6,v/v) with 0.1 eq. of SrCl2as cocatalyst at 708C. This strategy solved the problems of the hydrolysis of methyl glycosides, particularly the formation of large quantities of byproducts and poor yields,where there are no acceptable methods currently available. Acknowledgments
The authors are very grateful to the Natural Science Foundation of China (No. 21302141) and the Key Projects of Tianjin Science and Technology Support Plan (No. 10ZCKFSH01300) for financial support.
| [1] | Y.H. Shi, H.Q. Xu, B.N. Liu, et al., A facile synthesis of 6-deoxydapagliflozin, Monatsh. Chem. 144 (2013) 1903-1910. |
| [2] | B. Ron, S. Caryn, G. Irena, Synthesis of C-glucosides by reactions of glucosyl halides with organocuprates, J. Org. Chem. 53 (1988) 4026-4031. |
| [3] | J.J. Chiara, L. Encinas, B. Diaz, A study of polymer-supported bases for the solution phase synthesis of glycosyl trichloroacetimidates, Tetrahedron Lett. 46 (2005) 2445-2448. |
| [4] | K.J. Liao, X.F. Jin, X.B. Meng, et al., Synthesis of an antimetastatic tetrasaccharide β-D-Gal-(1→4)-β-D-GlcpNAc-(1→6)-α-D-Manp-(1→6)-β-D-Manp-OMe, Chin. Chem. Lett. 23 (2013) 1371-1374. |
| [5] | D.J. Leaver, A.B. Hughes, A. Polyzos, J.M. White, X-ray crystal structure determinations of galactosylacetylene building blocks, J. Carbohydr. Chem. 29 (2010) 379- 385. |
| [6] | J. Karl, N. Ghazi, M. Goeran, 2-(Trimethylsilyl)ethyl glycosides. Transformation into glycopyranosyl chlorides, J. Org. Chem. 55 (1990) 3181-3185. |
| [7] | J. Defaye, H. Driguez, B. Henrissat, et al., Stereochemistry of the hydrolysis of a,atrehalose by trehalase, determined by using a labelled substrate, Carbohydr. Res. 124 (1983) 265-274. |
| [8] | L. Nathalie, M. Chris, Synthesis of L-altrose and some derivatives, Eur. J. Org. Chem. 2012 (2012) 6260-6270. |
| [9] | M. Yamada, Y. Maeda, C.I. Someya, et al., Synthesis of endohedral metallofullerene glycoconjugates by carbene addition, Molecules 16 (2011) 9495-9504. |
| [10] | A. Presser, O. Kunert, I. Pötschger, High-yield syntheses of tetra-O-benzyl-a-Dglucopyranosyl bromide and tetra-O-pivaloyl-α-D-glucopyranosyl bromide and their advantage in the Koenigs-Knorr reaction, Monatsh. Chem. 137 (2006) 365- 374. |
| [11] | M.A. Fernández-Herrera, H. López-Muñoz, J.M. Hernández-Vázquez, et al., Synthesis and biological evaluation of the glycoside (25R)-3b,16b-diacetoxy-22- oxocholest-5-en-26-yl β-D-glucopyranoside: a selective anticancer agent in cervicouterine cell lines, Eur. J. Med. Chem. 46 (2011) 3877-3886. |
| [12] | S. Koto, N. Morishima, Y. Miyata, S. Zen, Preparation of 2,3,4,6-tetra-O-benzyl-Dmannose, Bull. Chem. Soc. Jpn. 49 (1976) 2639-2640. |
| [13] | D.G. Bourke, D.J. Collins, A.I. Hibberd, M.D. Mcieod, Enolic ortho esters. VI. A new ‘pyranose → cyclohexane' transformation via 1,6-dideoxy-1,1-ethylenedioxy- 2,3,4-tri-O-methyl-D-xylo-hex-5-enopyranose, Aust. J. Chem. 49 (1996) 425-434. |
| [14] | A.A. Bowers, M.A. Cinelli, W.J. Wever, Visible light mediated activation and Oglycosylation of thioglycosides, Org. Lett. 15 (2013) 30-33. |
| [15] | M. Matwiejuk, J. Thiem, New method for regioselective glycosylation employing saccharide oxyanions, Eur. J. Org. Chem. 2011 (2011) 5860-5878. |
| [16] | I.M. Pinilla, M.B. Martinez, J.A. Galbis, Synthesis of 2,3,4,5-tetra-O-methyl-Dglucono- 1,6-lactone as a monomer for the preparation of copolyesters, Carbohydr. Res. 338 (2003) 549-556. |
| [17] | R. Helleur, V.S. Rao, A.S. Perlin, Synthesis of 2,3,4,6-tetra-O-benzyl-L-idopyranose, Carbohydr. Res. 89 (1981) 83-90. |
| [18] | L. Kaesbeck, H. Kessler, Convenient syntheses of 2,3,4,6-tetra-O-alkylated Dglucose and D-galactose, Liebigs Ann. 1 (997) (1997) 169-174. |
| [19] | F. Charbonnier, S. Penades, A straightforward synthesis of 1-adamantylmethyl glycosides, and their binding to cyclodextrins, Eur. J. Org. Chem. 2004 (2004) 3650-3656. |
| [20] | R.U. Lemieux, U. Spohr, M. Bach, et al., Chemical mapping of the active site of the glucoamylase of Aspergillus niger, Can. J. Chem. 74 (1996) 319-335. |
| [21] | J.J. Verendel, T.L. Church, P.G. Anderson, Catalytic one-pot production of small organics from polysaccharides, Synthesis 2011 (2011) 1649-1677. |