Recent years have witnessed an unthinkable upsurge in potential utility of asymmetric organocatalysis as a tool for the synthesis of enantiopure molecules under mild,environmentally benign conditions [1]. Readily,available cinchona alkaloids, namely cinchonidine (CD),cinchonine (CN),quinine (QN) and quinidine (QD),enjoyed much interest as privileged organocatalysts effective in numerous mechanistically diverse enantioselective transformations [2]. The resulting (8S,9S) and (8R,9R)-9- amino-(9-deoxy) cinchona alkaloids are employed as attractive and efficient organocatalysts with excellent catalytic properties in asymmetric Aldol addition [3],Diels-Alder reaction [4],Friedel- Crafts reaction [5],Henry reaction [6],Mannich reaction [7], Michael addition [8],hydrogenation [9],epoxidation [10],1,3- dipolar cycloaddition [11],decarboxylation [12] and methanolytic desymmetrization [13]. In the aforementioned organocatalytic asymmetric reactions,the stereochemistry of catalytic products,to a great extent,generally depended on the configurations of the 8- and 9-stereogenic centers in 9-amino-(9-deoxy) cinchona alkaloids, although the experimental observations occasionally revealed the unexpected hint of enantioselectivity [14]. Considering the influence of stereochemical diversity of the organocatalyst on the stereochemistry of the products,(8S,9R) and (8R,9S)-9-amino-(9-deoxy) cinchona alkaloids possibly play the different and unthinkable role in determining the spatial structures of catalytic products. However,until now,(8S,9R) and (8R,9S)-9-amino-(9-deoxy) cinchona alkaloids have not been developed or received considerable attention. Based on the Mitsunobu reaction,in this report,four exotic pseudoenantiomers of (8S,9R) and (8R,9S)-9-amino-(9-deoxy) cinchona alkaloids were conveniently synthesized for the first time through the two conversions of the configurations at the 9-stereogenic centers in commercially available cinchona alkaloids (Scheme 1).

|
Download:
|
| Scheme 1.The synthetic route to 9-amino-(9-deoxy) cinchona alkaloids through the two inversions of configuration. | |
General procedure for 9-amino(9-deoxy) cinchona alkaloids: The epi-cinchona alkaloids (epi-CD,CN,QN and QD) (1.63 mmol) and triphenylphosphine (520 mg,1.95 mmol) were dissolved in 6 mL of anhydrous THF under an argon atmosphere and added dropwise 2 mL of diisopropyl azidocarboxylate (DIAD) (0.38 mL, 1.95 mmol) and 1 mL THF solution of diphenylphosphoryl azide (DPPA) (0.42 mL,1.95 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 12 h and 50 °C for 2 h and then triphenylphosphine (563 mg,2.12 mmol) added in one portion. After the gas evolution had ceased (about 2 h),the reaction mixture was cooled to room temperature,added 0.3 mL of water,stirred for another 3 h and evaporated under reduced pressure. The residue was dissolved in 10 mL of CH2Cl2 and added 5 mL of diluted hydrochloric acid (10%). The aqueous phase was washed with CH2Cl2 (3 × 10 mL),alkalinized with an excess of concentrated ammonia and extracted with CH2Cl2 (3 × 30 mL). The organic phase was dried over anhydrous Na2SO4. The concentrated organic phase was purified by silica gel column chromatography using CHCl3-MeOH (40:1,v/v) as an eluent to afford the title compounds as yellowish viscous oils.
CN-NH2: Yellowish viscous oil,α20D = -56.0 (c 0.30,EtOH). 1H NMR (300 MHz,CDCl3,Me4Si): d 8.89 (d,1 H,3J = 6.0 Hz,H-2'),8.21 (d,1 H,3J = 6.0 Hz,H-5'),8.12 (d,1 H,3J = 6.0 Hz,H-8'),7.70 (t,1 H, 3J = 6.0 Hz,H-7'),7.57 (t,1 H,3J = 6.0 Hz,H-60),7.41 (d,1 H, 3J = 3.0 Hz,H-3'),5.93-6.05 (m,1 H,H-10),5.12 (d,1 H,3J = 6.0 Hz, H-11a),5.08 (d,1 H,3J = 6.0 Hz,H-11b),4.81 (d,1 H,3J = 9.0 Hz,H- 9),3.18 (q,1 H,3J = 9.0 Hz,H-8),2.66-2.88 (m,4 H,H-6a,H-2-exo, H-6β,H-2-endo),2.25 (q,1 H,3J = 9.0 Hz,H-3),1.90-1.95 (m,3 H, NH2,H-4),1.80 (d,2 H,3J = 9.0 Hz,H-7a,H-7β),1.62 (q,2 H, 3J = 9.0 Hz,H-5). 13CNMR(75 MHz,CDCl3): d 150.0 (C-20),148.3 (C- 100),139.3 (C-4'),130.2 (C-10),129.1 (C-7'),128.8 (C-8'),126.5 (C- 90),126.3 (C-60),122.6 (C-5'),117.6 (C-3'),114.9 (C-11),60.4 (C-8), 52.1 (C-9),49.1 (C-2),48.1 (C-6),38.8 (C-3),29.4 (C-7),27.5 (C-4), 25.6 (C-5). Anal. Calcd. for C19H23N3: C,77.78; H,7.90; N,14.32. Found: C,77.75; H,9.93; N,14.27. MS: m/z 293.8 [M+H]+. QD-NH2: Yellowish viscous oil,α20D = -106.7 (c 0.79,EtOH). 1H NMR (300 MHz,CDCl3,Me4Si): d 8.72 (d,1 H,3J = 6.0 Hz,H-2'),8.01 (d,1 H,3J = 9.0 Hz,H-8'),7.42 (s,1 H,H-5'),7.31-7.37 (m,2 H,H-7', H-3'),5.93-6.04 (m,1 H,H-10),5.04-5.12 (m,2 H,H-11),4.73 (d,1 H,3J = 9.0 Hz,H-9),3.95 (s,3H,H-110),3.13 (q,1 H,3J = 9.0 Hz,H-8), 2.65-2.92 (m,4 H,H-6a,H-2-exo,H-6β,H-2-endo),2.24 (q,1 H, 3J = 9.0 Hz,H-3),2.13 (s,2 H,NH2),1.86 (s,1 H,H-4),1.77 (q,2 H, 3J = 6.0 Hz,H-7a,H-7β),1.60 (q,2 H,3J = 9.0 Hz,H-5). 13C NMR (75 MHz,CDCl3): δ 157.5 (C-60),149.3 (C-20),147.6 (C-100),144.4 (C-1'),140.2 (C-4'),131.6 (C-8'),127.6 (C-9'),121.2 (C-3'),118.0 (C-7'),114.5 (C-11),101.0 (C-5'),60.6 (C-8),55.4 (C-2),52.8 (C-11'), 49.3 (C-9),48.4 (C-6),39.2 (C-3),27.7 (C-7),26.1 (C-5),25.2 (C-4). Anal. Calcd. for C20H25N3O: C,74.27; H,7.79; N,12.99; O,4.95. Found: C,74.34; H,7.81; N,12.96; O,4.96. MS: m/z 324.7 [M+H]+.
CD-NH2: Yellowish viscous oil,α20D = -55.6 (c 1.63,EtOH). 1H NMR (300 MHz,CDCl3,Me4Si): δ 8.89 (d,1 H,3J = 6.0 Hz,H-2'),8.22 (d,1 H,3J = 6.0 Hz,H-5'),8.13 (d,1 H,3J = 9.0 Hz,H-8'),7.70 (t,1 H, 3J = 6.0 Hz,H-7'),7.57 (t,1 H,3J = 6.0 Hz,H-60),7.42 (1 d,H, 3J = 6.0 Hz,H-3'),5.86-5.98 (m,1 H,H-10),5.03-5.09 (m,2 H,H- 11),4.76 (d,1 H,3J = 9.0 Hz,H-9),3.23 (q,1 H,3J = 9.0 Hz,H-8), 2.96-3.07 (m,2 H,H-6a,H-2-exo),2.66 (d,1 H,3J = 15.0 Hz,H-6β), 2.55 (td,1 H,3J 1= 12.0 Hz,3J 2= 6.0 Hz,H-2-endo),2.28 (s,3 H,NH2, H-3),2.16 (m,1 H,H-4),1.90 (d,1 H,H-7β),1.68 (td,1 H, 3J 1= 12.0 Hz,3J 2= 3.0 Hz,H-7a),1.50-1.56 (q,2 H,3J = 9.0 Hz,H-5). 13C NMR (75 MHz,CDCl3): δ 150.3 (C-20),148.6 (C-100),141.7 (C- 40),130.5 (C-10),129.3 (C-7'),128.9 (C-8'),126.6 (C-9'),126.5 (C- 60),122.6 (C-5'),118.0 (C-3'),114.5 (C-11),60.6 (C-8),56.1 (C-2), 53.4 (C-9),41.8 (C-6),39.6 (C-3),27.7 (C-7),27.6 (C-4),26.4 (C-5). Anal. Calcd. for C19H23N3: C,77.78; H,7.90; N,14.32. Found: C, 77.72; H,9.98; N,14.29. MS: m/z 293.7 [M+H]+.
QN-NH2: Yellowish viscous oil,α20D = -42.3 (c 1.76,EtOH). 1HNMR (300 MHz,CDCl3,Me4Si): δ 8.74 (d,1 H,3J = 6.0Hz,H-2'),8.02 (d,1 H,3J = 12.0 Hz,H-8'),7.44 (d,1 H,3J = 3.0Hz,H-5'),7.38 (1 H,d, 3J = 3.0Hz,H-7'),7.35 (d,1H,3J = 6.0Hz,H-3'),5.87-5.99 (1 H,m,H- 10),5.03-5.09 (m,2 H,H-11),4.63 (d,1 H,3J = 9.0 Hz,H-9),3.96 (s,3 H,H-110),3.21 (q,1 H,3J = 9.0 Hz,H-8),2.95-3.07(m,2 H,H-6a,H-2- exo),2.66 (d,1 H,3J = 12.0 Hz,H-6β),2.56 (1 H,td,3J 1= 12.0Hz, 3J 2= 3.0 Hz,H-2-endo),2.28 (s,1 H,H-3),2.15 (td,1 H,3J 1= 9.0Hz 3J 2= 3.0 Hz,H-4),1.90 (s,3 H,NH2,H-7β,),1.67 (td,1H,3J 1= 15.0Hz, 3J 2= 3.0Hz,H-7a),1.52 (2 q,H,3J = 9.0Hz,H-5). 13C NMR (75MHz, CDCl3): d 157.7 (C-60),149.2 (C-20),147.8 (C-100),144.7 (C-10),141.8 (C-4'),131.9 (C-8'),127.6 (C-9'),121.1 (C-3'),118.3 (C-7'),114.4 (C- 11),101.1 (C-5'),60.5 (C-8),56.1 (C-2),55.6 (C-11'),53.7 (C-9),41.9 (C-6),39.6 (C-3),27.8 (C-7),27.7 (C-4),26.5 (C-5). Anal. Calcd. for C20H25N3O: C,74.27; H,7.79; N,12.99; O,4.95. Found: C,74.32; H, 7.80; N,12.97; O,4.98. MS: m/z 324.9 [M+H]+. 3. Results and discussion
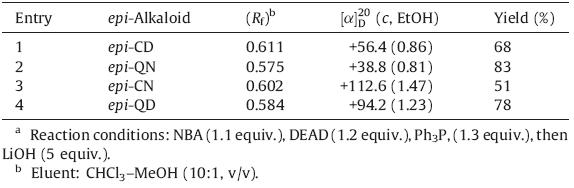
The first conversion of cinchona alkaloids to 9-epimers of cinchona alkaloids was achieved through one-pot inversion of Mitsunobu esterification-saponification [15]. Four cinchona alkaloids (CD,CN,QN and QD) were esterified with 4-nitrobenzoic acid (NBA) and subsequently treated in situ with aqueous lithium hydroxide solution. The corresponding 9-epi-cinchona alkaloids (epi-CD,CN,QN and QD) were easily purified by flash column chromatography using CHCl3/t-BuOMe (3:1,v/v) as an eluent to remove Ph3PO and diethyl hydrazine-1,2-dicarboxylate,and then CHCl3/MeOH/Et3N (40:1:1,v/v/v) to obtain the target compounds of analytical purity in 51%-83% yield,which were identified by 1H- NMR,13C NMR,α20D and Rf (Table 1).
| Table 1 Inversion of cinchona alkaloids by Mitsunobu esterification/saponification.a |
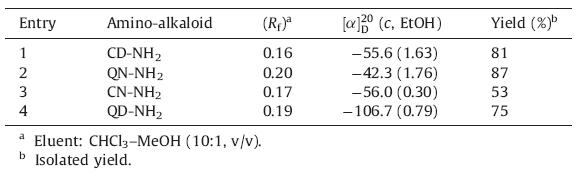
According to the reported synthetic procedure [16],four (8S,9R) and (8R,9S)-9-amino(9-deoxy) cinchona alkaloids (CD-NH2,CN-NH2,QN-NH2,QD-NH2) were conveniently prepared in good yields (53%-87%) [17]. The key step was the Mitsunobu reaction that led to the C9-azido compound by an SN2 mechanism. The reduction is performed in situ by adding triphenylphosphane. Hydrolysis of the intermediate aminophosphorane yielded free amine. The pure 9-amino(9-deoxy) cinchona alkaloids (CD-NH2, CN-NH2,QN-NH2 and QD-NH2) were easily separated by silica gel flash column chromatography using CHCl3/MeOH (40:1,v/v) as an eluent to afford yellowish viscous oils,whose structures were identified by 1H NMR,13C NMR,1H COSY 2D NMR,1H NOESY 2D NMR,α20D and Rf (Table 2). It is noteworthy that the α20D values of CD-NH2,CN-NH2,QN-NH2 and QD-NH2 were converted from positive to negative due to the inversions of the configurations at the 9-hydroxyl groups of epi-CD,CN,QN and QD.
| Table 2 IInversion of epi-cinchona alkaloids to 9-amino(9-deoxy) cinchona alkaloids by Mitsunobu reaction. |
Herein,we provided the direct evidence for conformational preference of 9-amino(9-deoxy) cinchona alkaloids (CD-NH2,CNNH2, QN-NH2 and QD-NH2) through a combination of 1H NOESY 2D NMR and computational methods. Full geometric optimization calculations were performed in chloroform using the polarized continuum model (PCM) with B3LYP functional and 6-31G(d) basis set,and their minimum energy structures were shown in the Supporting information. For the identification of conformers,the interring NOEs between the quinoline and quinuclidine protons and NOEs involving H8 and H9 are most useful. Pointedly,four 9- amino(9-deoxy) cinchona alkaloids appeared to exhibit the hindered rotations around the C4'-C9 and C9-C8 bonds and to favor only a narrow range of the available conformational space of the molecule. The 1H NOESY 2D NMR of CN-NH2 displayed only three cross-peaks related to aromatic hydrogen,indicating close interactions between the H5'-H9,H5'-H8,and H3'-H6 pairs of atoms. These data mainly supported the minimum energy conformation of CN-NH2,and elucidated that CN-NH2 favored the open conformation (Fig. 1). Although the NOESY spectrum of CD-NH2,QN-NH2 and QD-NH2 did not directly support their minimum energy conformations,but the favored conformations could be obtained through molecular rotations around the C4'-C9 and C9-C8 bonds from their minimum energy structures,in which the H5'-H9 and H3'-H8 pairs of atoms showed close NOE interactions with the closed conformations [18]. Furthermore, 1H NMR and 13CNMR spectra of (8S,9R)-CD-NH2 differed from that of (8S,9S)-9-amino-(9-deoxy) cinchona alkaloids (epi-CD-NH2) because of their opposite configurations of carbon atoms at C9-position. The mixture of 50 wt% (8S,9R)-CD-NH2 and 50 wt% (8S,9S)-epi-CD-NH2 showed the more sophisticated 1H NMR and 13C NMR spectra than their own shown in the Supporting information,which provided the indirect evidence to elucidate the different configurations at the C9-position between (8S,9R)- CD-NH2 and (8S,9S)-epi-CD-NH2.

|
Download:
|
| Fig. 1.1H NOESY 2D NMR and minimum energy conformation of CN-NH2. | |
In summary,four exotic (8S,9R) and (8R,9S)-9-amino(9-deoxy) cinchona alkaloids were conveniently synthesized for the first time through the two conversions of the configurations at the 9- stereogenic centers of commercially available cinchona alkaloids. Their catalytic performances in various asymmetric reactions are under investigation. Acknowledgments
Research support from the National Science Foundation of
China (No. 21071116) and Chongqing Scientific Foundation,China
(No. 2010BB4126).
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in
the online version,at
| [1] | (a) J. Seayad, B. List, Asymmetric organocatalysis, Org. Biomol. Chem. 3 (2005) 719-724; (b) C.F. Barbas ⅡI, Organocatalysis lost: modern chemistry, ancient chemistry, and an unseen biosynthetic apparatus, Angew. Chem. Int. Ed. 47 (2008) 42-47; (c) W. Notz, F. Tanaka, C.F. Barbas ⅡI, Enamine-based organocatalysis with proline and diamines: the development of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels-Alder reactions, Acc. Chem. Res. 37 (2004) 580-591; (d) H. Chen, R. Jiang, Q.F. Wang, et al., Synthesis of chiral dihydrofuran compounds by thiourea derivatives-catalyzed "interrupted" Feist-Bénary reaction, Chin. Chem. Lett. 21 (2010) 167-170. |
| [2] | (a) S.K. Tian, Y.G. Chen, J.F. Hang, et al., Asymmetric organic catalysis with modified cinchona alkaloids, Acc. Chem. Res. 37 (2004) 621-631; (b) S.J. Connon, Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts, Chem. Commun. (2008) 2499-2510; (c) T. Marcelli, H. Hiemstra, Cinchona alkaloids in asymmetric organocatalysis, Synthesis 8 (2010) 1229-1279. |
| [3] | (a) J. Zhou, V. Wakchaure, P. Kraft, B. List, Primary-amine-catalyzed enantioselective intramolecular aldolizations, Angew. Chem. Int. Ed. 47 (2008) 7656-7658; (b) B.L. Zheng, Q.Z. Liu, C.S. Guo, X.L. Wang, L. He, Highly enantioselective direct Aldol reaction catalyzed by cinchona derived primary amines, Org. Biomol. Chem. 5 (2007) 2913-2915; (c) J.Q. Zhou, J.W. Wan, X.B. Ma, W. Wang, Copolymer-supported heterogeneous organocatalyst for asymmetric Aldol addition in aqueous medium, Org. Biomol. Chem. 10 (2012) 4179-4185; (d) W.Wang, X.B.Ma, J.W.Wan, J. Cao, Q. Tang, Preparation and confinement effect of a heterogeneous 9-amino-9-deoxy-epi-cinchonidine organocatalyst for asymmetric Aldol addition in aqueous medium, Dalton Trans. 41 (2012) 5715-5726. |
| [4] | R.P. Singh, K. Bartelson, Y. Wang, et al., Enantioselective Diels-Alder reaction of simple α,β-unsaturated ketones with a cinchona alkaloid catalyst, J. Am. Chem. Soc. 130 (2008) 2422-2423. |
| [5] | (a) H.M. Li, Y.Q. Wang, L. Deng, Enantioselective Friedel-Crafts reaction of indoles with carbonyl compounds catalyzed by bifunctional cinchona alkaloids, Org. Lett. 8 (2006) 4063-4065; (b) G. Bartoli, M. Bosco, A. Carlone, et al., Organocatalytic asymmetric Friedel- Crafts alkylation of indoles with simple α,β-unsaturated ketones, Org. Lett. 9 (2007) 1403-1405. |
| [6] | P. Hammar, T. Marcelli, H. Hiemstra, F. Himo, Density functional theory study of the Cinchona thiourea-catalyzed Henry reaction: mechanism and enantioselectivity, Adv. Synth. Catal. 349 (2007) 2537-2548. |
| [7] | T.Y. Liu, H.L. Cui, J. Long, et al., Organocatalytic and highly stereoselective direct vinylogous Mannich reaction, J. Am. Chem. Soc. 129 (2007) 1878-1879. |
| [8] | (a) P.F. Li, Y.C. Wang, X.M. Liang, J.X. Ye, Asymmetric multifunctional organocatalytic Michael addition of nitroalkanes to α,β-unsaturated ketones, Chem. Commun. 28 (2008) 3302-3304; (b) B. Vakulya, S. Varga, A. Csámpai, T. Soós, Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts, Org. Lett. 7 (2005) 1967-1969; (c) J.P. Malerich, K. Hagihara, V.H. Rawal, Chiral squaramide derivatives are excellent hydrogen bond donor catalysts, J. Am. Chem. Soc. 130 (2008) 14416-14417. |
| [9] | H.Y. Jiang, C.F. Yang, C. Li, et al., Heterogeneous enantioselective hydrogenation of aromatic ketones catalyzed by cinchona- and phosphine-modified iridium catalysts, J. Angew. Chem. Int. Ed. 47 (2008) 9240-9244. |
| [10] | (a) X.W. Wang, C.M. Reisinger, B. List, Catalytic asymmetric epoxidation of cyclic enones, J. Am. Chem. Soc. 130 (2008) 6070-6071; (b) X.J. Lu, Y. Liu, B.F. Sun, B. Cindric, L. Deng, Catalytic enantioselective peroxidation of α,β-unsaturated ketones, J. Am. Chem. Soc. 130 (2008) 8134-8135; (c) Q.F. Wang, H. Chen, P. Liu, et al., Asymmetric epoxidation of alpha, betaenones catalyzed by chiral amine salts, Chin. J. Org. Chem. 29 (2009) 1617-1620; (d) X.D. Liu, X.L. Bai, X.P. Qiu, L.X. Gao, Asymmetric phase-transfer mediated epoxidation of alpha, beta-enones using dendritic catalysts derived from cinchona alkaloids, Chin. Chem. Lett. 16 (2005) 975-978. |
| [11] | W. Chen, W. Du, Y.Z. Duan, et al., Enantioselective 1,3-dipolar cycloaddition of cyclic enones catalyzed by multifunctional primary amines: beneficial effects of hydrogen bonding, Angew. Chem. Int. Ed. 46 (2007) 7667-7670. |
| [12] | H. Brunner, M.A. Baur, a-Amino acid derivatives by enantioselective decarboxylation, Eur. J. Org. Chem. (2003) 2854-2862. |
| [13] | (a) S.H. Oh, H.S. Rho, J.W. Lee, et al., A highly reactive and enantioselective bifunctional organocatalyst for the methanolytic desymmetrization of cyclic anhydrides: prevention of catalyst aggregation, Angew. Chem. Int. Ed. 47 (2008) 7872-7875; (b) H.S. Rho, S.H. Oh, J.W. Lee, et al., Bifunctional organocatalyst for methanolytic desymmetrization of cyclic anhydrides: increasing enantioselectivity by catalyst dilution, Chem. Commun. (2008) 1208-1210. |
| [14] | M. Bartók, Unexpected inversions in asymmetric reactions: reactions with chiral metal complexes, chiral organocatalysts, and heterogeneous chiral catalysts, Chem. Rev. 110 (2010) 1663-1705. |
| [15] | Ł. Sidorowicz, J. Skarżewski, Easy access to 9-epimers of cinchona alkaloids: onepot inversion by Mitsunobu esterification-saponification, Synthesis 5 (2011) 708-710. |
| [16] | H. Brunner, J. Bügler, B. Nuber, Enantioselective catalysis 98. Preparation of 9- amino(9-deoxy)cinchona alkaloids, Tetrahedron: Asymmetry 6 (1995) 1699- 1702. |
| [17] | C.G. Oliva, A.M.S. Silva, D.I.S.P. Resende, F.A.A. Paz, J.A.S. Cavaleiro, Highly enantioselective 1,4-Michael additions of nucleophiles to unsaturated aryl ketones with organocatalysis by bifunctional cinchona alkaloids, Eur. J. Org. Chem. (2010) 3449-3458. |
| [18] | H. Brunner, P. Schmidt, M. Prommesberger, Enantioselective catalysis. Part 133: Conformational analysis of amides of 9-amino(9-deoxy)epicinchonine, Tetrahedron: Asymmetry 11 (2000) 1501-1502. |