b College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China
The catalytic oxidation of sp3 C-H bond adjacent to a nitrogen atom to form a new carbon-carbon or carbon-hetero bond has been widely applied in organic synthesis [1]. Using the iminium ions generated from the direct oxidation of tertiaryaminesin situ, Strecter reaction,Mannich reaction,aza-Henry reactions and so on have been successfully realized [2]. Recently,visible light photoredox catalysis aroused great interest due to its green chemistry characteristics [3]. Many metal complexes and organic dyes were frequently used as photocatalysts in photoredox chemistry [4, 5]. In particular,several highly efficient chemical transformations were established using Ir or Ru photocatalysts [5]. The photocatalytic oxidation of tertiaryamines is a very important research topic.
Kobayashi reported that a Ru complex,in the presence of visible light,catalyzed the cross oxidative coupling of 1,3-diphenylpropane-1,3-dione and tertiary aliphatic amines to form methylenebridged bis-1,3-dicarbonyl compounds [6, 7]. However,in the course of our study,it was found that without metal photocatalyst, 1,3-diphenylpropane-1,3-dione and N,N-dimethylcyclohexylamine reacted smoothly to give the methylene-bridged bis-1,3-dicarbonyl compounds even using only visible light,albeit in very low yield. Although the exact mechanism was unclear,the observation may be ascribed to the differences in reaction conditions between Kobayashi’s and ours. 2. Experimental
All reactions were carried out without the exclusion of air or moisture. Toluene,cyclohexane,DCE,DCM,DMSO,DMF,EtOAc, 1,4-dioxane and MeCN were analytical reagents and used directly as received.N,N-dimethylcyclohexylamine,N,N-dimethylbutylamine,N,N-dimethylbenzylamine,N,N-dimethylaniline,N,N,N',N' -tetramethylethane-1,2-diamine,N-methyldiisopropylamine,Nmethyldicyclohexylamine were purchased from the Sigma- Aldrich company and used directly as received. 1,3-Dicarbonyl compounds were prepared according to literature procedures [8]. 1H NMR and 13C NMR spectra were recorded at ambient temperature in CDCl3on a Bruker AMX-500 MHz instrument with TMS as an internal standard. Low-resolution MS was obtained using EI or ESI ionization. HRMS was obtained using ESI ionization.
General procedure for the preparation of compounds2:toa mixture of 1,3-diphenylpropane-1,3-dione1a(0.5 mmol) and 9/1 (v/v) DMSO/H2O (2 mL) in a 10 mL two-necked round-bottom flask was addedN-methyl-dicyclohexanamine (0.25 mmol). The resulting mixture was stirred under 1 atm of O2(balloon) for 24 h at ambient temperature under the irradiation by three 45 W white fluorescent bulbs (Arrow BHSL 45) at a distance of 5 cm. Then the reaction mixture was washed three times with dilute hydrochloric acid. Purification was done by column chromatography on silica gel (200-300 mesh) using petroleum ether and ethyl acetate (5:1- 10:1) as the eluent to give the pure product 2a.
Synthesis and characterization of all products are described in Supporting information. 3. Results and discussion
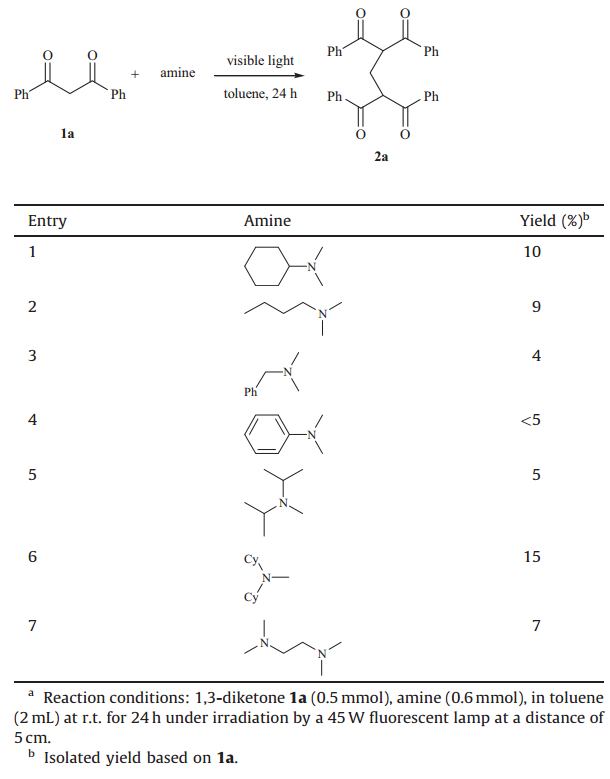
Our initial studies focused on the visible light-mediated reaction of 1,3-diphenylpropane-1,3-dione1aandN,N-dimethylcyclohexylamine. Unfortunately,the reaction proved to be very sluggish and the starting material could not be fully consumed within 30 h (Table 1,entry 1). VariousN-methyl tertiaryamines as potential methylene donors were examined. To our disappointment,all aliphaticN-methyl tertiaryamines tried led to the desired product with very low yields. WhenN,N-dimethylaniline was introduced,the reaction resulted in a complex mixture (Table 1, entry 4).
|
|
However,it was interesting to find that the photocatalytic reaction ofN-methyldicyclohexylamine was significantly faster, compared with other N-methyl tertiaryamines. This reaction could occur in all solvents tried. It was gratifying that,in contrast to other solvents (Table 2),the desired product was obtained in 70% isolated yield after 24 h using DMSO as a solvent (Table 2, entry 5).
|
|
Based on these results,various conditions were evaluated. It was found that strong light emission could accelerate the reaction. Interestingly,use of H2O as a solvent also resulted in high yields (Table 3,entry 3). The mixed solvent of DMSO and H2O gave reasonable result as well. Importantly,no conversion was observed when the reaction was conducted in the dark (Table 3,entries 8 and 9). Under O2,the reaction was more rapid than under air,while a very slow background reaction was observed under N2. These observations indicated that oxygen was essential in this reaction.
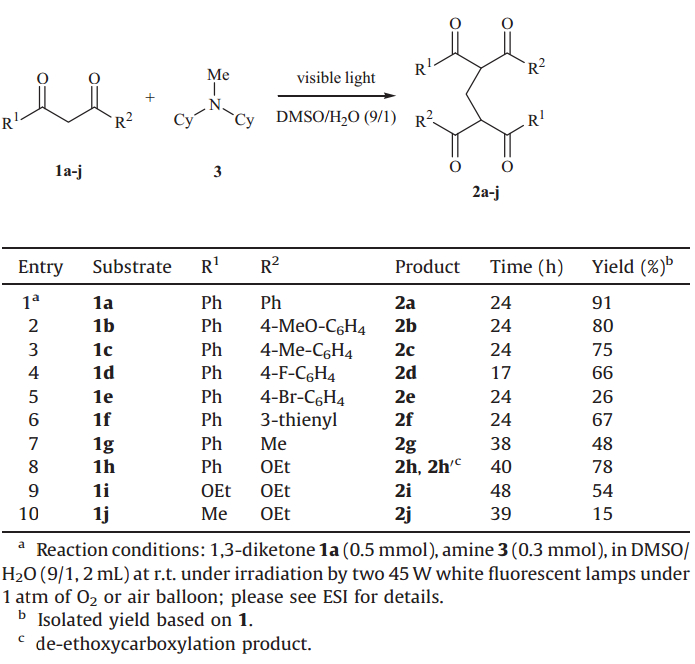
Under the optimized conditions,the scope of this photonmediated coupling reaction was investigated. In general a variety of different dicarbonyl nucleophiles (Table 4),including aryl and alkyl 1,3-diketones,b-ketoesters and 1,3-diester,could react withN-methyl-dicyclohexylamine. Substrates containing substituents like Me and OMe on the aryl rings underwent the reaction smoothly (Table 4,entries 2 and 3). For substrates with halogenated phenyl rings,a fluoro appeared to be more favorable than a bromo and some unidentified compounds formed in certain reactions (Table 4,entries 4 and 5). Substrates with alkyl ketone moieties gave lower yields of the product (Table 4,entry 7). When b-ketoesters were utilized as substrates,the reactions resulted in modest yields (Table 4,entries 8-10). In the case of ethyl 3-oxo-3-phenylpropanoate 1h,the desired product 2h was obtained in 39% yield,accompanied by 39% of a de-ethoxycarboxylated product 2h'.
|
|
|
|
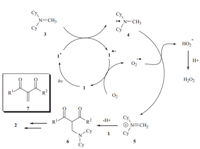
A plausible mechanism for the reaction is proposed in Scheme 1. We reasoned that the 1,3-dicarbonyl compounds are effective photo mediators. Upon irradiation,1is excited providing 1*,which is then reductively quenched by3to produce a radical anion of 1 and amine radical cation 4 via a single electron transfer process.1 is regenerated with the assistance of dioxygen,and the resulting oxygen radical anion abstracts a proton in the methyl group in radical cation 4 to generate the iminium intermediate 5. Subsequently,nucleophilic trapping of 5 with 1 results in the oxidative coupling product 5. The desired product 2 is formed by either a direct nucleophilic substitution reaction or a tandem Cope elimination and Michael additionviaintermediate 7.
|
Download:
|
| Scheme 1. Proposed reaction mechanism. | |
In summary,without any other photocatalyst,we have developed a useful protocol for the synthesis of methylenebridged bis-1,3-dicarbonyl compounds in aqueous media using visible light photoredox catalysis. In contrast to the similar reactions reported in recent literature,the mechanism of this reaction needs more experimental studies. Further investigations into the mechanism of this reaction under visible light are currently underway. Acknowledgment
The authors are grateful to Zhejiang Provincial Natural Science Foundation of China (No. LY12B02017). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.021.
| [1] | (a) S.I. Murahashi, D.Z. Zhang, Ruthenium catalyzed biomimetic oxidation in organic synthesis inspired by cytochrome P-450, Chem. Soc. Rev. 37 (2008) 1490-1501; (b) C.J. Li, Cross-dehydrogenative coupling (CDC): exploring C-C bond formations beyond functional group transformations, Acc. Chem. Res. 42 (2009) 335-344; (c) C.J. Li, The development of catalytic nucleophilic additions of terminal alkynes in water, Acc. Chem. Res. 43 (2010) 581-590; (d) C.S. Yeung, V.M. Dong, Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds, Chem. Rev. 111 (2011) 1215-1292; (e) C. Zhang, C.H. Tang, N. Jiao, Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process, Chem. Soc. Rev. 41 (2012) 3464-3484. |
| [2] | (a) A.G. Condie, J.C. González-Gómez, C.R.J. Stephenson, Visible-light photoredox catalysis: aza-Henry reactions via C-H functionalization, J. Am. Chem. Soc. 132 (2010) 1464-1465; (b) M. Rueping, C. Vila, R.M. Koenigs, K. Poscharny, D.C. Fabry, Dual catalysis: combining photoredox and Lewis base catalysis for direct Mannich reactions, Chem. Commun. 47 (2011) 2360-2362; (c) M. Rueping, S.Q. Zhu, R.M. Koenigs, Visible-light photoredox catalyzed oxidative Strecker reaction, Chem. Commun. 47 (2011) 12709-12711; (d) D.B. Freeman, L. Furst, A.G. Condie, C.R.J. Stephenson, Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light, Org. Lett. 14 (2012) 94-97; (e) G.L. Zhao, C. Yang, L. Guo, et al., Visible light-induced oxidative coupling reaction: easy access to Mannich-type products, Chem. Commun. 48 (2012) 2337-2339; (f) M. Rueping, R.M. Koenigs, K. Poscharny, et al., Dual catalysis: combination of photocatalytic aerobic oxidation and metal catalyzed alkynylation reactions-C-C bond formation using visible light, Chem. Eur. J. 18 (2012) 5170-5174; (g) J. Xuan, Y. Cheng, J. An, et al., Visible light-induced intramolecular cyclization reactions of diamines: a new strategy to construct tetrahydroimidazoles, Chem. Commun. 47 (2011) 8337-8339; (h) S.Q. Zhu, M. Rueping, Merging visible-light photoredox and Lewis acid catalysis for the functionalization and arylation of glycine derivatives and peptides, Chem. Commun. 48 (2012) 11960-11962. |
| [3] | (a) K. Zeitler, Photoredox catalysis with visible light, Angew. Chem. Int. Ed. 48 (2009) 9785-9789; (b) T.P. Yoon, M.A. Ischay, J. Du, Visible light photocatalysis as a greener approach to photochemical synthesis, Nat. Chem. 2 (2010) 527-532; (c) J.M.R. Narayanam, C.R.J. Stephenson, Visible light photoredox catalysis: applications in organic synthesis, Chem. Soc. Rev. 40 (2011) 102-113; (d) F. Teplý, Photoredox catalysis by [Ru(bpy)3]2+ to trigger transformations of organic molecules. Organic synthesis using visible-light photocatalysis and its 20th century roots, Collect. Czech. Chem. Commun. 76 (2011) 859-917; (e) L. Shi, W.J. Xia, Photoredox functionalization of C-H bonds adjacent to a nitrogen atom, Chem. Soc. Rev. 41 (2012) 7687-7697; (f) Y.M. You, W. Nam, Photofunctional triplet excited states of cyclometalated Ir(ⅡI) complexes: beyond electroluminescence, Chem. Soc. Rev. 41 (2012) 7061- 7084; (g) J. Xuan, W.J. Xiao, Visible-light photoredox catalysis, Angew. Chem. Int. Ed. 51 (2012) 6828-6838; (h) Y.M. Xi, H. Yi, A.W. Lei, Synthetic applications of photoredox catalysis with visible light, Org. Biomol. Chem. 11 (2013) 2387-2403; (i) C.K. Prier, D.A. Rankic, D.W.C. MacMillan, Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis, Chem. Rev. 113 (2013) 5322-5363. |
| [4] | (a) H. Schmaderer, P. Hilgers, R. Lechner, B. König, Photooxidation of benzyl alcohols with immobilized flavins, Adv. Synth. Catal. 351 (2009) 163-174; (b) A. Berlicka, B. König, Porphycene-mediated photooxidation of benzylamines by visible light, Photochem. Photobiol. Sci. 9 (2010) 1359-1366; (c) R. Lechner, S. Kummel, B. König, Visible light flavin photo-oxidation of methylbenzenes, styrenes and phenylacetic acids, Photochem. Photobiol. Sci. 9 (2010) 1367-1377; (d) M. Neumann, S. Füldner, B. König, K. Zeitler, Metal-free, cooperative asymmetric organophotoredox catalysis with visible light, Angew. Chem. Int. Ed. 50 (2011) 951-954; (e) K. Ohkubo, K. Mizushima, R. Iwata, S. Fukuzumi, Selective photocatalytic aerobic bromination with hydrogen bromide via an electron-transfer state of 9- mesityl-10-methylacridinium ion, Chem. Sci. 2 (2011) 715-722; (f) W.H. Zhong, Y.N. Cui, S.M. Li, Y.P. Jia, J.M. Yin, The carbonylation of phenyl bromide and its derivatives under visible light irradiation, Chin. Chem. Lett. 23 (2012) 29-32; (g) D.S. Hamilton, D.A. Nicewicz, Direct catalytic anti-markovnikov hydroetherification of alkenols, J. Am. Chem. Soc. 134 (2012) 18577-18580; (h) J.M.C. Grandjean, D.A. Nicewicz, Synthesis of highly substituted tetrahydrofurans by catalytic polar-radical-crossover cycloadditions of alkenes and alkenols, Angew. Chem. Int. Ed. 52 (2013) 3967-3971; (i) M. Riener, D.A. Nicewicz, Synthesis of cyclobutane lignans via an organic single electron oxidant-electron relay system, Chem. Sci. 4 (2013) 2625-2629; (j) D.J. Wilger, N.J. Gesmundo, D.A. Nicewicz, Catalytic hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes via an organic photoredox system, Chem. Sci. 4 (2013) 3160-3165; (k) T.M. Nguyen, D.A. Nicewicz, Anti-Markovnikov hydroamination of alkenes catalyzed by an organic photoredox system, J. Am. Chem. Soc. 135 (2013) 9588-9591; (l) A.J. Perkowski, D.A. Nicewicz, Direct catalytic anti-Markovnikov addition of carboxylic acids to alkenes, J. Am. Chem. Soc. 135 (2013) 10334-10337. |
| [5] | (a) D.A. Nicewicz, D.W.C. MacMillan, Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes, Science 322 (2008) 77-80; (b) P.V. Pham, D.A. Nagib, D.W.C. MacMillan, Photoredox catalysis: a mild, operationally simple approach to the synthesis ofa-trifluoromethyl carbonyl compounds, Angew. Chem. Int. Ed. 50 (2011) 6119-6122; (c) M.A. Ischay,M.E.Anzovino, J.Du, T.P. Yoon, Efficient visible light photocatalysis of[2+2] enone cycloadditions, J. Am. Chem. Soc. 130 (2008) 12886-12887; (d) Z. Lu, T.P. Yoon, Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer, Angew. Chem. Int. Ed. 51 (2012) 10329-10332; (e) J.M.R. Narayanam, J.W. Tucker, C.R.J. Stephenson, Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction, J. Am. Chem. Soc. 131 (2009) 8756-8757; (f) J.D. Nguyen, E.M. D'Amato, J.M.R. Narayanam, C.R.J. Stephenson, Engaging unactivated alkyl, alkenyl and aryl iodides in visible-light-mediated free radical reactions, Nat. Chem. 4 (2012) 854-859; (g) C.J. Wallentin, J.D. Nguyen, P. Finkbeiner, C.R.J. Stephenson, Visible light-mediated atom transfer radical addition via oxidative and reductive quenching of photocatalysts, J. Am. Chem. Soc. 134 (2012) 8875-8884; (h) M. Rueping, D. Leonori, T. Poisson, Visible light mediated azomethine ylide formation-photoredox catalyzed [3 + 2] cycloadditions, Chem. Commun. 47 (2011) 9615-9617; (i) Y. Miyake, Y. Ashida, K. Nakajima, Y. Nishibayashi, Visible-light-mediated addition of α-aminoalkyl radicals generated from a-silylamines to α,β-unsaturated carbonyl compounds, Chem. Commun. 48 (2012) 6966-6968; (j) T. Hering, D.P. Hari, B. Ko¨nig, Visible-light-mediated a-arylation of enol acetates using aryl diazonium salts, J. Org. Chem. 77 (2012) 10347-10352; (k) M. Chen, Z.T. Huang, Q.Y. Zheng, Visible light-induced 3-sulfenylation of Nmethylindoles with arylsulfonyl chlorides, Chem. Commun. 48 (2012) 11686- 11688; (l) M.H. Larraufie, R. Pellet, L. Fensterbank, et al., Visible-light-induced photoreductive generation of radicals from epoxides and aziridines, Angew. Chem. Int. Ed. 50 (2011) 4463-4466; (m) Y. Yasu, T. Koike, M. Akita, Visible-light-induced synthesis of a variety of trifluoromethylated alkenes from potassium vinyltrifluoroborates by photoredox catalysis, Chem. Commun. 49 (2013) 2037-2039. |
| [6] | W.J. Yoo, A. Tanoue, S. Kobayashi, Aerobic oxidation of a tertiary aliphatic amine under visible-light photocatalysis: facile synthesis of methylene-bridged bis-1,3- dicarbonyl compounds, Chem. Asian J. 7 (2012) 2764-2767. |
| [7] | (a) D.R. Hwang, B.J. Uang, A modified Mannich-type reaction catalyzed by VO(acac) 2, Org. Lett. 4 (2002) 463-466; (b) Y.S. Hon, T.R. Hsu, C.Y. Chen, et al., Dibromomethane as one-carbon source in organic synthesis: microwave-accelerated a-methylenation of ketones with dibromomethane and diethylamine, Tetrahedron 59 (2003) 1509-1520; (c) H.J. Li, Z.H. He, X.W. Guo, et al., Iron-catalyzed selective oxidation of N-methyl amines: highly efficient synthesis of methylene-bridged bis-1,3-dicarbonyl compounds, Org. Lett. 11 (2009) 4176-4179; (d) R. Balamurugan, S. Manojveer, Gold/copper-catalyzed activation of the aciform of nitromethane in the synthesis of methylene-bridged bis-1,3-dicarbonyl compounds, Chem. Commun. 47 (2011) 11143-11145. |
| [8] | A.G. Hu, W.B. Lin, Ru-catalyzed asymmetric hydrogenation of a-phthalimide ketones and 1,3-diaryl diketones using 4,40-substituted BINAPs, Org. Lett. 7 (2005) 455-458. |