b University of Chinese Academy of Sciences, Beijing 100049, China
The genus Isodon,which includes about 150 species,is a cosmopolitan and important genus of the family Lamiaceae and has attracted considerable attention as a prolific source of diterpenoids with diverse structures and biological activities [1, 2]. Isodon sculponeatus (Vaniot) Kudo,a perennial herb,is distributed widely in southern China,and has been used as a folk medicine for treatment of dysentery and beriberi [3, 4]. Previous phytochemical investigations of this species proved that it was a rich source of bioactive diterpenoids,mainly ent-kauranoids [5- 15],such as sculponeatin I [11],sculponin A [12],and sculponeatin O [15]. In our efforts to seek structurally interesting and potential bioactive diterpenoids from the genus Isodon,we investigated the chemical constituents of the aerial parts of I. sculponeatus collected from Muli County of Sichuan Province,China,and have found a series of ent-kaurane diterpenoids,of which some have antitumor and anti-inflammatory activities [16]. A continued phytochemical investigation on this plant led to the isolation of an additional one new 6,7-seco-ent-kaurane diterpenoid,sculponin T (1),together with four known analogs,sculponeatin J (2) [10],sculponeatin K (3) [10],sculponeatin C (4) [6],and sculponeatin Q (5) [15] (Fig. 1). Herein,we report the isolation and structure elucidation of the new compound,as well as the in vitro cytotoxicity evaluation of all isolates obtained against five human tumor cell lines (HL-60, SMMC-7721,A-549,MCF-7,and SW-480),and the inhibitory activity against NO production in LPS-stimulated RAW264.7 cells of compounds 1,2,and 4.

|
Download:
|
| Fig. 1.Structures of compounds 1-5. | |
The aerial parts of I. sculponeatus were collected in August 2011 and identified by Prof. Xi-Wen Li,Kunming Institute of Botany. A voucher specimen (KIB 20110813) has been deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China,Kunming Institute of Botany,Chinese Academy of Sciences. 2.2. Extraction and isolation
The dried and powdered aerial parts of I. sculponeatus (8 kg) were extracted with 70% aqueous acetone (30 L) four times (two days each time) at room temperature,then filtered. The filtrate was evaporated under reduced pressure and then partitioned between EtOAc and H2O. The EtOAc soluble portion (700 g) was subjected to silica gel CC (3 kg,100-200 mesh),eluted with a CHCl3-Me2CO gradient system (1:0-0:1) that afforded fractions A-G. The fractions were then decolorized using MCI gel and eluted with 90% MeOH-H2O. Fraction B (120 g) was chromatographed via silica gel CC (200-300 mesh),eluted with CHCl3-MeOH gradient (150:1- 1:1) to yield fractions B1-B5. Compound 4 (139 μg) was crystallized from fraction B5. Fraction B1 was purified by repeated chromatography over silica gel CC (petroleum ether-Me2CO gradient,12:1-0:1),followed by semipreparative HPLC (30% MeCN-H2O),to yield compounds 5 (40 μg),2 (47 μg),and 3 (19 μg). Fraction D (100 g) was subjected to RP-18 CC (MeOH-H2O gradient,10%-100%) to give fractions D1-D6. Then fraction D2 was separated by CC on silica gel (200-300 mesh),eluted with CHCl3- MeOH (gradient system: 100:1-1:1),to give compound 1 (4 μg). 2.3. The cytotoxicity assay
The human tumor cell lines HL-60,SMMC-7721,A-549,MCF- 7,and SW-480 were used,which were obtained from ATCC (Manassas,VA,USA). All the cells were cultured in RPMI-1640 or DMEM medium (Hyclone,Logan,UT,USA),supplemented with 10% fetal bovine serum (Hyclone) at 37℃ in a humidified atmosphere with 5% CO2. Cell viability was assessed by conducting colorimetric measurements of the amount of insoluble formazan formed in living cells based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2- (4-sulfopheny)-2H-tetrazolium (MTS) (Sigma,St. Louis,MO, USA) [17]. Briefly,100 μL of adherent cells were seeded into each well of a 96-well cell culture plate and allowed to adhere for 12 h before drug addition,while suspended cells were seeded just before drug addition,both with an initial density of 1 × 105 cells/mL in 100 μL medium. Each tumor cell line was exposed to the test compound at various concentrations in triplicate for 48 h,with cisplatin and paclitaxel (Sigma) as positive controls. After the incubation,MTS (100 μg) was added to each well,and the incubation continued for 4 h at 37℃. The cells were lysed with 100 μL of 20% SDS-50% DMF after removal of 100 μL medium. The optical density of the lysate was measured at 490 nm in a 96-well microtiter plate reader (Bio-Rad 680). The IC50 value of each compound was calculated by the Reed and Muench’s method [18]. 2.4. Nitric oxide production in RAW264.7 macrophages
Murine monocytic RAW264.7 macrophages were dispensed into 96-well plates (2 × 105 cells/well) containing RPMI 1640 medium (Hyclone) with 10% FBS under a humidified atmosphere of 5% CO2 at 37℃. After 24 h preincubation,cells were treated with serial dilutions of the compounds,with the maximum concentration of 25 μmol/L,in the presence of 1 μg/mL LPS for 18 h. Each compound was dissolved in DMSO and further diluted in medium to produce different concentrations. NO production in each well was assessed by adding 100 μL of Griess reagent (Reagent A and Reagent B,respectively,Sigma) to 100 μL of each supernant from LPS (Sigma)-treated or LPS- and compound-treated cells in triplicate. After 5 min incubation,the absorbance was measured at 570 nm with a 2104 Envision Multilabel Plate Reader (Perkin- Elmer Life Sciences,Inc.,Boston,MA,USA). MG-132 was used as a positive control [19]. 3. Results and discussion
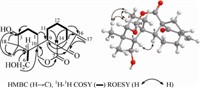
Compound 1,[α]D26.0 + 12:1 (c 0.06,MeOH),UV (MeOH) λmax (log ε): 232 (3.67),201 (3.62) nm,obtained as a white,amorphous powder,displayed a molecular ion peak at m/z 348.1933 ([M]+, calcd. 348.1937) in its HREIMS,in accordance with a molecular formula C20H28O5. Its IR absorptions at 3430,1739,1710,and 1643 cm-1 implied the existence of OH,C=O,and C=C groups. The 1H NMR spectrum (Table 1) indicated the presence of two tertiary methyl groups (δH 1.34,0.98),and two olefinic protons (δH 5.91, 5.26). The 13C NMR and DEPT data (Table 1) suggested that 1 was a diterpenoid with a total of 20 carbons,consisting of two methyls, eight methylenes (two oxygenated and one olefinic),four methines (one oxygenated),and six quaternary carbons (two carbonyl and one olefinic). Analysis of the HSQC and 1H-1H COSY spectra (Fig. 2) revealed the spin systems for the molecular fragments of C-1-C-2- C-3,C-5-C-6,and C-9-C-11-C-12-C-13-C-14. Their connectivity was deduced from the HMBC experiment. In the HMBC spectrum (Fig. 2),Me-18 and Me-19 correlated to C-3 (δC 74.6,d) and C-5 (δC 46.0,d),and H-5 (δH 2.36,overlap) correlated to C-4 (δC 38.9,s), suggesting that C-4 quaternary carbon was connected to Me-18 and Me-19,and C-3 was connected to C-5 through C-4. The HMBC correlations fromH2-6 (δH 3.95,m) to C-4,C-5,and C-10 (δC 42.5,s), established the attachment of an oxygenated methylene carbon (C- 6) to C-5. Correlations from H-9 (δH 3.15,dd,J = 14.7,5.0 Hz) to C-1 (δC 19.8,t) and C-10,and from H2-20 (δH 4.91,d,J = 8.3 Hz) to C-1, C-9 (δC 45.3,d),and C-10 suggested that C-10 was connected with C-1,C-9,and C-20. In addition,HMBC correlations from H-11 (δH 1.85,m) and H-13 (δH 2.85,m) to C-8 (δC 58.5,s),from H-14 (δH 2.51,dd,J = 12.3,4.5 Hz) to C-9,from H-17 (δH 5.26,br s) to C-13 (δC 35.6,d),C-15 (δC 203.6,s),and C-16 (δC 151.4,s),and from H-9 to C- 15 suggested that C-8,C-9,and C-11-C-14 constituted the sixmembered ring C,and allowed the connection of C-13 and C-16, and of C-8 and C-15 to form a five-membered ring D. Thus,the basis skeleton of 1 was characteristic of a 6,7-seco-ent-kaurane diterpenoid. Finally,the presence of an OH group at C-3 was evident from the HMBC correlations from H-2 (δH 1.75,overlap), Me-18,and Me-19 to C-3.
|
Download:
|
| Fig. 2.Key HMBC,1H-1H COSY,and ROESY correlations of 1. | |
| Table 1 NMR data of sculponin T (1) (C5D5N,TMS,d in ppm,J in Hz).a,b |
The relative configurations of 1 were established by the ROESY experiment (Fig. 2). The ROESY correlation between H-5 and H-9β was in agreement with the β-orientation of H-5. The α-orientation for CH2-20 was evident from the ROESY correlations from H2-20 to H-2α and Me-19α. In addition,the HO-3 of 1 was assigned to be β-oriented for the upfield shifts of C-1 (δ -19.4 ppm) and C-5 (δ -10.5 ppm) compared with those of C-1 and C-3 in macrocalyxoformin D [20],which was caused by the γ-steric compression effect between HO-3 and H-1β and between HO-3 and H-5β. Thus, the structure of compound 1 was determined as 3β,6-dihydroxy- 6,7-seco-ent-kaur-16-en-15-one-7,20-olide and given the name sculponin T.
1H NMR,13C NMR,DEPT,HSQC,HMBC,COSY,ROESY,HREIMS, IR and UV spectra of compound 1 are supplied in Supporting information.
Compounds 2-5 were identified by comparison of their physical constant data with data in the literature [6, 10, 15].
Considering the cytotoxicity of diterpenoids previously isolated from the plants of the genus Isodon [1],all isolates were evaluated for their in vitro cytotoxicity against five human tumor cell lines (HL-60,SMMC-7721,A-549,MCF-7,and SW-480) according to a previously described procedure [17]. The results are given in Table 2. Compound 2,with the existence of an a-exomethylenecyclopentanone group,exhibited the highest potency against all the five cancer cell lines with IC50 values of 2.9,3.3,3.3,2.8,and 1.8 μmol/L,respectively. Compounds 1 and 4,with the existence of an a-exomethylene-cyclopentanone group,showed higher cytotoxicity against the five human tumor cell lines,compared with those of compounds 3 and 5 without an a-exomethylenecyclopentanone group,which were inactive in the tested system (IC50 > 40 μmol/L). This finding suggests that the a-exomethylene- cyclopentanone group might be important in mediating the cytotoxicity of ent-kauranoids,and the result is in good agreement with conclusions of previous structure-activity relationship research [1].
| Table 2 IC50 values (mmol/L) of diterpenoids from I. sculponeatus for human tumor cell lines. |
In addition,due to the folk use of I. sculponeatus [3, 4],and since NO is an essential component of the host innate immune and inflammatory responses to a variety of pathogens [21],the antiinflammatory assay in LPS-stimulated RAW264.7 cells was carried out on compounds 1,2,and 4. As a result,compound 2 exhibited strong inhibitory activity against LPS-induced NO production with IC50 value of 3.3 μmol/L,while compounds 1 and 4 showed weak inhibitory activity with respective IC50 value of 18.6 μmol/L and 21.2 μmol/L. At the highest concentration used,none of the tested compounds exhibited inhibitory activities,which suggested that the inhibitory activities against NO production in LPS-stimulated RAW264.7 cells were not induced by the cytotoxicity of the compounds tested. 4. Conclusion
In summary,one new 6,7-seco-ent-kaurane diterpenoid and another four known analogs were isolated from the aerial parts of I. sculponeatus. All isolates were evaluated for their in vitro cytotoxicity against HL-60,SMMC-7721,A-549,MCF-7,and SW- 480 cell lines. Compound 2 exhibited significant cytotoxic activity against all the cell lines with IC50 values ranging from 1.8 μmol/L to 3.3 μmol/L,while compounds 1 and 4 showed moderate cytotoxicity. Selected compounds were evaluated for their inhibitory activity on NO production in LPS-stimulated RAW264.7 cells,and compounds 1,2,and 4 showed intriguing activity. Acknowledgments
This project was supported financially by the National Natural Science Foundation of China (Nos. 21322204 and 81172939),the NSFC-Joint Foundation of Yunnan Province (No. U1302223),the Reservation-talent Project of Yunnan Province (No. 2011CI043), and the West Light Foundation of the Chinese Academy of Sciences (Jian-Xin Pu). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.041.
| [1] | H.D. Sun, S.X. Huang, Q.B. Han, Diterpenoids from Isodon species and their biological activities, Nat. Prod. Rep. 23 (2006) 673-698. |
| [2] | H.D. Sun, Y.L. Xu, B. Jiang, Diterpenoids from Isodon Species, Science Press, Beijing, 2001, p. 4. |
| [3] | Compiling Groups of Compilation of Countrywide Herbal Medicine of China, Compilation of Countrywide Herbal Medicine of China, People's Medical Publishing House, Beijing, 1996, p. 853. |
| [4] | Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita, Flora Reipublicae Popularis Sinica, Science Press, Beijing, 1977, p. 504. |
| [5] | X.R. Wang, Z.Q. Wang, J.G. Dong, A new diterpene from Huanghua-xiangchacai (Rabdosia sculponeata), Zhongcaoyao 13 (1982) 11-12. |
| [6] | H.D. Sun, Z.W. Lin, Y.L. Xu, et al., Structures of sculponins A, B and C, three new diterpenoids having unique acetal structures from Rabdosia sculponeata, Heterocycles 24 (1986) 1-4. |
| [7] | R.P. Zhang, H.J. Zhang, H.D. Sun, et al., Diterpenoids from Rabdosia sculponeata, Chin. Chem. Lett. 2 (1991) 293-296. |
| [8] | M.H. Yang, Q.S. Zhao, H.D. Sun, et al., Studies on diterpenoids of Isodon sculponeata, Zhongcaoyao 32 (2001) 397-399. |
| [9] | B. Jiang, H. Yang, H.D. Sun, et al., Two new ent-kauranoids from Isodon sculponeata, Chin. Chem. Lett. 13 (2002) 1083-1086. |
| [10] | B. Jiang, S.X. Mei, H.D. Sun, et al., Diterpenoids from Isodon sculponeatus, Chin. J. Chem. 20 (2002) 887-890. |
| [11] | B. Jiang, A.J. Hou, H.D. Sun, et al., Cytotoxic ent-kaurane diterpenoids from Isodon sculponeata, Planta Med. 68 (2002) 921-925. |
| [12] | L.M. Li, G.Y. Li, H.D. Sun, et al., Sculponins A-C, three new 6,7-seco-ent-kauranoids from Isodon sculponeatus, Tetrahedron Lett. 48 (2007) 9100-9103. |
| [13] | F. Wang, X.M. Li, J.K. Liu, New terpenoids from Isodon sculponeata, Chem. Pharm. Bull. 57 (2009) 525-527. |
| [14] | (a) L.M. Li, G.Y. Li, H.D. Sun, et al., Ent-kaurane and cembrane diterpenoids from Isodon sculponeatus and their cytotoxicity, J. Nat. Prod. 72 (2009) 1851-1856; (b) L.M. Li, J.X. Pu, H.D. Sun, et al., A new phenylethanoid glycoside from Isodon sculponeatus, Chin. Chem. Lett. 22 (2011) 961-963. |
| [15] | X. Li, J.X. Pu, H.D. Sun, et al., 6,7-seco-ent-Kaurane diterpenoids from Isodon sculponeatus with cytotoxic activity, Chem. Biodivers. 7 (2010) 2888-2896. |
| [16] | H.Y. Jiang, W.G. Wang, H.D. Sun, et al., Enmein-type 6,7-seco-ent-kauranoids from Isodon sculponeatus, J. Nat. Prod. 76 (2013) 2113-2119. |
| [17] | A. Monks, D. Scudiero, P. Cronise, et al., Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines, J. Natl. Cancer Inst. 83 (1991) 757-766. |
| [18] | L.J. Reed, H. Muench, A simple method of estimating fifty percent endpoints, Am. J. Hyg. 27 (1983) 493-497. |
| [19] | J.T. Fan, J. Su, N.H. Tan, et al., Rubiyunnanins C-H, cytotoxic cyclic hexapeptides from Rubia yunnanensis inhibiting nitric oxide production and NF-kB activation, Bioorg. Med. Chem. 18 (2010) 8226-8234. |
| [20] | H.D. Sun, Y.L. Xu, B. Jiang, Diterpenoids from Isodon Species, Science Press, Beijing, 2001, p. 312. |
| [21] | N.L. McCartney-Francis, X. Song, D.E. Mizel, et al., Selective inhibition of inducible nitric oxide synthase exacerbates erosive joint disease, J. Immunol. 166 (2001) 2734. |