The incorporation of fluorine atom(s) or fluorine-containing moieties into organic molecules would affect their lipophilicity and spatial structure,thus emanating unique physical,chemical and biological properties,which can lead to potential applications in materials,medicinal,pharmaceutical and agrochemical sciences [1]. In particular,the replacement of metabolically active hydrogen atoms with fluorine atoms (or CF3) is commonly used in contemporary medicinal chemistry to improve metabolic stability, bioavailability and protein-drug interactions [2]. This strategy has been illuminated in the organocatalytic asymmetric synthesis of fluorinated molecules and several important methods have been developed in the last decade [3].
Pyrano[2, 3-c]pyrazoles are a large class of heterocyclic compounds possessing many important biological activities,such as analgesic,anti-inflammatory,anti-tumor,insecticidal activities and so on [4]. Since the first reaction for the synthesis of pyrano[2, 3-c]pyrazole derivatives from 3-methyl-1-phenylpyrazolin-5-one and tetracyanoethylene was reported by Junek in 1973 [5a],a large number of efficient synthetic methods to construct dihydropyrano[2, 3-c]pyrazole derivatives have been developed [5]. Very recently,Zhao has reported the first organocatalyzed enantioselective method for the synthesis of these compounds using the cinchona alkaloid as the catalyst [6]. However,to the best of our knowledge,no chiral fluorinated dihydropyrano[2, 3-c]pyrazoles have been reported up to date [7]. Herein,based on some previous studies in our group [8],we would like to present an efficient catalyst system for the cascade Michael addition- cyclization using diaminocyclohexane-thioureas [9] as a bifunctional catalyst providing a series of novel chiral fluorinated dihydropyrano[2, 3-c]pyrazoles. 2. Experimental
1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz,respectively,with TMS as an internal standard.19F NMR spectra were recorded at 282 MHz with CFCl3 as an external standard. IR spectra were recorded in cm-1 . Melting points were determined on an apparatus,which was not corrected. All solvents were distilled prior to use unless otherwise noted. All reactions sensitive to moisture or oxygen were conducted under an atmosphere of nitrogen or argon.
To a mixture of 1 (0.1 mmol,1.0 equiv.) and catalyst 3c (0.01 mmol,0.1 equiv.) in CHCl3(2 mL) was added2(0.12 mmol, 1.2 equiv.). Then the reaction solution was vigorously stirred at 08C and monitored by TLC analysis. After the reaction was complete,the mixture was concentrated and purified by flash column chromatography on silica gel (petroleum ether/EtOAc as the eluent) to furnish the corresponding product 4. The data of compounds4a-pcan be found in Supporting information. 3. Results and discussion
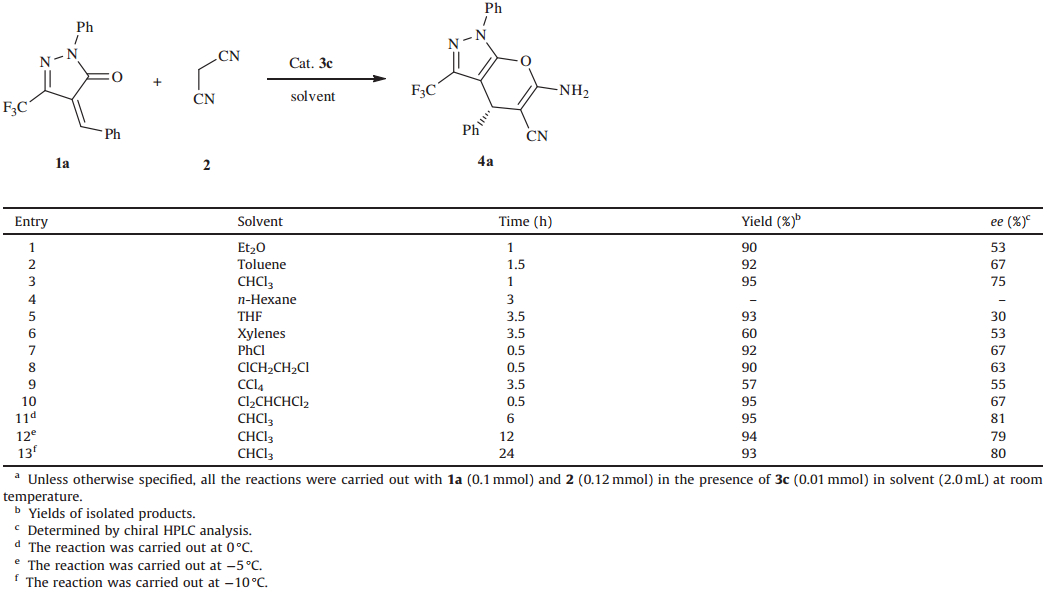
Initially,the Takemoto’s catalyst3a(Fig. 1) was investigated in the model reaction between (Z)-4-benzylidene-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-5(4H)-one (1a) and malononitrile (2)in dichloromethane (DCM) at room temperature. The reaction gave the desired cyclic product4ain an excellent yield; however,a poor enantioselectivity (57% ee) was also observed (Table 1,entry 1). In order to enhance the enantioselectivity,several other diaminocyclohexane-thiourea catalysts with weaker H-bond donating ability on the thiourea were screened. As expected,the yields that were more correlative with the tertiary amine altered little with these catalysts but higher enantioselectity could be obtained with catalysts3b-f(entries 2-7). With thec-hexyl substituted catalyst 3h,a lower eevalue was obtained (entry 8). Encouraged by the above improvement made with the modified diaminocyclohexane-thiourea catalysts,additional catalysts3h-mderived from amino acids and the cinchona alkaloid were also synthesized and evaluated in this reaction. We found that the cinchona alkaloidderived bifunctional thiourea catalysts3iand3jboth gave poor enantioselectivity (entries 9 and 10) probably due to their strong base effect and with the amino acid-based catalysts3k,3land3m; similar inferior results were also obtained. For all of the reactions, the desired products could be obtained in high yields (>90%) in no more than 50 min.
|
Download:
|
| Fig. 1.Thiourea catalysts evaluated in this study. | |
| Table 1 Screening of the catalysts.a |
Then the reactions in various solvents were screened. The results were summarized in Table 2. Generally,the reactions proceeded smoothly in most solvents providing4ain excellent yields except in those that the substrates have poor solubility in such as n-hexane,xylenes and CCl4 (entries 4,6,9). Further investigation showed that the reaction worked the best in chloroform,which gave the desired product in 95% yield and 75%ee(entry 3). Lowering the reaction temperature from room temperature to 08C increased theee value to 81% while the excellent yield was maintained (entry 11). However,even lower temperatures brought no improvement in enantioselectivity but led to a prolonged reaction time (entry 13). As a consequence,the optimal condition for this Michael addition-cyclization was obtained with 10 mol % of3cin CHCl3at 08C.
| Table 2 Optimization of the reactions in various solvents under different temperatures.a |
Having established the optimal conditions for the cascade Michael addition-cyclization,we next explored the scope of the reaction and the representative results are listed in Table 3. For pyrazoles with different R1substituents,excellent yields and good eevalues could be generally obtained regardless of the electronic nature or positions of the substituents on the phenyl group of R1 except the substrates with a strong electron-withdrawing nitro group orortho-substituents (entries 1-8). This reaction was also tolerant with a heteroaromatic ring by giving the product 4gin good yield and enantioselectivity (entry 9). A similar substituent effect was observed while changing the substituents on R2. Although slightly lowereevalues were obtained with this type of substrates,good enantioselectivity could also be obtained with electro-donating substituents (entries 10-15).
| Table 3 Investigation of reaction scope.a |
Notably,although in a prolonged reaction time,the nonfluorinated substrate1qalso proceeded efficiently to give the desired product4qin excellent yields with slightly lowereevalues (Scheme 1),which provides an easy access to this kind of important intermediates.

|
Download:
|
| Scheme 1.Reaction of the non-fluorinated substrate 1q. | |
In an effort to realize the goal of generating biologically interesting products,further transformations of the chiral pyrano[2, 3-c]pyrazole products4obtained above were then investigated (Scheme 2). With aluminum chloride as a catalyst [10],the Friedla¨nder reaction of the pyrano[2, 3-c]pyrazole4fwith cyclohexanone afforded the heterocyclic product5in 70% yield without sacrificing the stereo-integrity. The absolute configuration of5was then confirmed by X-ray crystallography to be S [11] and the absolute configuration of product4was determined in the same manner (Scheme 2). It should be noted that these types of heterocyclic compounds with pyranopyridine scaffold are known to possess potential biological activities,such as antiallergic,anti inflammatory,and estrogenic properties [12]. In addition,the compounds containing benzopyrano[2, 3-b]pyridine structure also exhibit anti-proliferative,cancer chemopreventive and antibacterial (including anti-tubercular) activities [13].

|
Download:
|
| Scheme 2.Transformation of the product 4f. | |
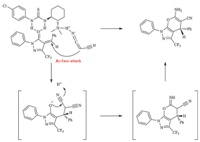
In light of the previous studies and the configuration obtained,a plausible reaction pathway was proposed in Scheme 3 [7]. The bifunctional thiourea catalyst is not only as a base to deprotonate the malononitrile by the tertiary amine moiety but also activates the carbonyl group of 4-aryliden-5-pyrazolones by forming two hydrogen bonds using the N-Hs. When R1 or R2 contained electron-donating groups,more powerful hydrogen bonds were formed and herein higheevalues were achieved. The mechanism could also explain why loweevalues were obtained withortho substituent on R2; the big steric hindrance made the hydrogen bonds weaker.
|
Download:
|
| Scheme 3.Proposed catalytic mechanism of the asymmetric Michael addition–cyclization reaction. | |
In summary,we have developed an asymmetric organocatalytic cascade Michael addition-cyclization reaction using bifunctional diaminocyclohexane-thiourea catalysts. The reactions gave the attractive products in excellent yields and good enantioselectivity under mild reaction conditions,making it a novel and valuable method for the construction of potential biologically active functionalized fluorinated dihydropyano[2, 3-c]pyrazoles,which are also important intermediates in medicinal chemistry and can be valuable for the pharmaceutical industry. Acknowledgments
This work was supported by National Basic Research Program of China (973 Program,No. 2010CB833200),the National Natural Science Foundation of China (Nos. 21032006,203900502, 20532040,21290180),Science and Technology Commission of Shanghai Municipality (No. 11XD1406400). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.034
| [1] | (a) K. Mikami, Y. Itoh, Y.M. Yamamaka, Fluorinated carbonyl and olefinic compounds: basic character and asymmetric catalytic reactions, Chem. Rev. 104 (2004) 1-16; (b) B.E. Smart, Fluorine substituent effects (on bioactivity), J. Fluor. Chem. 109 (2001) 3-11; (c) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications, Wiley-VCH, Weinheim, 2004; (d) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Blackwell, Oxford, 2009. |
| [2] | (a) S. Purser, P.R. Moore, S. Swallow, V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev. 37 (2008) 320-330; (b) K. Müller, C. Faeh, F. Diederich, Fluorine in pharmaceuticals: looking beyond intuition, Science 317 (2007) 1881-1886; (c) W.K. Hagmann, The many roles for fluorine in medicinal chemistry, J. Med. Chem. 51 (2008) 4359-4369. |
| [3] | (a) V.A. Brunet, D. O'Hagan, Catalytic asymmetric fluorination comes of age, Angew. Chem. Int. Ed. 47 (2008) 1179-1182; (b) R. Smits, C.D. Cadicamo, K. Burger, B. Koksch, Synthetic strategies to a-trifluoromethyl and a-difluoromethyl substituted α-amino acids, Chem. Soc. Rev. 37 (2008) 1727-1739; (c) G.K.S. Prakash, P. Beier, Construction of asymmetric fluorinated carbon centers, Angew. Chem. Int. Ed. 45 (2006) 2172-2174; (d) P.M. Pihko, Enantioselective-fluorination of carbonyl compounds: organocatalysis or metal catalysis? Angew. Chem. Int. Ed. 45 (2006) 544-547; (e) M. Oestreich, Strategies for catalytic asymmetric electrophilic halogenation of carbonyl compounds, Angew. Chem. Int. Ed. 44 (2005) 2324-2327; (f) H. Ibrahim, A. Togni, Enantioselective halogenation reactions, Chem. Commun. (2004) 1147-1155; (g) J.A. Ma, D. Cahard, Asymmetric fluorination, trifluoromethylation and perfluoroalkylation, Chem Rev. 104 (2004) 6119-6146. |
| [4] | (a) S.C. Kuo, L.J. Huang, H. Nakamura, Studies on heterocyclic compounds. 6. Synthesis and analgesic and antiinflammatory activities of 3,4-dimethylpyrano[ 2,3-c]pyrazol-6-one derivatives, J. Med. Chem. 27 (1984) 539-544; (b) J.L. Wang, D. Liu, Z.J. Zhang, et al., Structure-based discovery of an organic compound that binds bcl-2 protein and induces apoptosis of tumor cells, Proc. Natl. Acad. Sci. USA 97 (2000) 7124-7129; (c) N. Foloppe, L.M. Fisher, R. Howes, et al., Identification of chemically diverse chk1 inhibitors by receptor-based virtual screening, Bioorg. Med. Chem. 14 (2006) 4792-4802. |
| [5] | (a) H. Junek, H. Aigner, Synthesen mit Nitrilen, XXXV. Reaktionen von tetracyanäthylen mit heterocyclen, Chem. Ber. 106 (1973) 914-921; (b) J.F. Zhou, S.J. Tu, H.Q. Zhu, S.J. Zhi, A facile one pot synthesis of pyrano[2,3- c]pyrazole derivatives under microwave irradiation, Synth. Commun. 32 (2002) 3363-3366; (c) A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, et al., Cross-condensation of derivatives of cyanoacetic acid and carbonyl compounds. Part 1: Singlestage synthesis of 10-substituted 6-amino-spiro-4-(piperidine-40)-2H,4H-pyrano[ 2,3-c]pyrazole-5-carbonitriles, Tetrahedron 59 (2003) 7491-7496; (d) D.Q. Shi, J. Mou, Q.Y. Zhuang, et al., Three-component one-pot synthesis of 1, 4-dihydropyrano[2,3-c]pyrazole derivatives in aqueous Media, Synth. Commun. 34 (2004) 4557-4563; (e) T.S. Jin, A.Q. Wang, Z.L. Cheng, J.S. Zhang, T.S. Li, A clean and simple synthesis of 6-amino-4-aryl-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole in water, Synth. Commun. 35 (2005) 137-143; (f) Z.J. Ren, W.G. Cao, W.Q. Tong, Z. Jin, Solvent-free, one-pot synthesis of pyrano[2,3-c]pyrazole derivatives in the presence of KF 2H2O by Grinding, Synth. Commun. 35 (2005) 2509-2513; (g) Y.Q. Peng, G.H. Song, R.L. Dou, Surface cleaning under combined microwave and ultrasound irradiation: flash synthesis of 4H-pyrano[2,3-c]pyrazoles in aqueous media, Green Chem. 8 (2006) 573-575; (h) F. Lehmann, M. Holm, S. Laufer, Three-component combinatorial synthesis of novel dihydropyrano[2,3-c]pyrazoles, J. Comb. Chem. 10 (2008) 364-367; (i) H. Sheibani, M. Babaie, Three-component reaction to form 1,4-dihydropyrano[ 2,3-c]pyrazol-5-yl cyanides, Synth. Commun. 40 (2009) 257-265; (j) A.S. Nagarajan, B.S.R. Reddy, Synthesis of substituted pyranopyrazoles under neat conditions via a multicomponent reaction, Synlett (2009) 2002-2004; (k) Y.M. Litvinov, A.A. Shestopalov, L.A. Rodinovskaya, A.M. Shestopalov, New convenient four-component synthesis of 6-amino-2,4-dihydropyrano[2,3-c]pyrazol- 5-carbonitriles and one-pot synthesis of 60-aminospiro[(3H)-indol-3,40-pyrano[ 2,3-c]pyrazol]-(1H)-2-on-50-carbonitriles, J. Comb. Chem. 11 (2009) 914-919; (l) R. Balaskar, S. Gavade, D. Mane, Greener approach towards the facile synthesis of 1,4-dihydropyrano[2,3-c]pyrazol-5-yl cyanide derivatives at room temperature, Chin. Chem. Lett. 21 (2010) 1175-1179; (m) H. Mecadon, M.R. Rohman, I. Kharbangar, et al., l-Proline as an efficient catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3- methyl- 2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in water, Tetrahedron Lett. 52 (2011) 3228-3231. |
| [6] | (a) S. Gogoi, C.G. Zhao, Organocatalyzed enantioselective synthesis of 6-amino-5- cyanodihydropyrano[2,3-c]pyrazoles, Tetrahedron Lett. 50 (2009) 2252-2255; (b) S. Muramulla, C.G. Zhao, A new catalytic mode of the modularly designed organocatalysts (mdos): enantioselective synthesis of dihydropyrano[2,3-c]pyrazoles, Tetrahedron Lett. 52 (2011) 3905-3908. |
| [7] | H.F. Zohdi, A.H.H. Elghandour, N.M. Rateb, M.M.M. Sallam, Reactions with 5-trifluoromethyl-2, 4-dihydropyrazol-3-one derivatives: a new route for the synthesis of fluorinated polyfunctionally substituted pyrazole and pyrano[2,3- c]pyrazole derivates, J. Chem. Res. 12 (1992) 3015-3025. |
| [8] | (a) X.S. Wang, C.W. Zheng, S.L. Zhao, et al., Organocatalyzed Friedel-craft-type reaction of 2-naphthol with b,g-unsaturated a-keto ester to form novel optically active naphthopyran derivatives, Tetrahedron: Asymmetry 19 (2008) 2699-2704; (b) Y.Q.Yang, G.Zhao, OrganocatalyzedhighlyenantioselectiveMichael additions of malonates to enones by using novel primary-secondary diamine catalysts, Chem. Eur. J. 14 (2008) 10888-10891; (c) S.L. Zhao, C.W. Zheng,G. Zhao, Enantioselective synthesis of multifunctionalized 4H-pyran derivatives using bifunctional thiourea-tertiary amine catalysts, Tetrahedron: Asymmetry 20 (2009) 1046-1051; (d) C.W. Zheng, Y.W. Li, H.F.Wang, et al., Highly efficient asymmetric epoxidation of electron-deficient, b-enones and related applications to organic synthesism, Adv. Synth. Catal. 351 (2009) 1685-1691; (e) P. Li, Z. Chai, S.L. Zhao, et al., Highly enantio- and diastereoselective synthesis of a-trifluoromethyldihydropyrans using a novel bifunctional piperazine-thiourea catalyst, Chem. Commun. (2009) 7369-7371; (f) H.F. Cui, Y.Q. Yang, Z. Chai, et al., Enantioselective synthesis of functionalized fluorinated cyclohexenones via Robinson annulation catalyzed by primary- secondary diamines, J. Org. Chem. 75 (2010) 117-122; (g) H.F. Cui, P. Li, X.W.Wang, et al., Highly enantioselective synthesis ofa-fluoro-anitro esters via organocatalyzed asymmetric Michael addition, Tetrahedron 67 (2011) 312-317; (h) H.F. Cui, P. Li, X.W. Wang, S.Z. Zhu, G. Zhao, Asymmetric Michael addition of afluoro- a-phenylsulfonyl ketones to nitroolefins catalyzed by phenylalanine-based bifunctional thioureas, J. Fluor. Chem. 133 (2012) 120-126. |
| [9] | (a) T. Okino, Y. Hoashi, Y. Takemoto, Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts, J. Am. Chem. Soc. 125 (2003) 12672-12673; (b) T. Okino, Y. Hoashi, T. Furukawa, X.N. Xu, Y. Takemoto, Enantio- and diastereoselective Michael reaction of 1,3-dicarbonyl compounds to nitroolefins catalyzed by a bifunctional thiourea, J. Am. Chem. Soc. 127 (2005) 119-125; (c) T. Inokuma, Y. Hoashi, Y. Takemoto, Thiourea-catalyzed asymmetric Michael addition of activated methylene compounds to α,β-unsaturated imides: dual activation of imide by intra- and intermolecular hydrogen bonding, J. Am. Chem. Soc. 128 (2006) 9413-9419; (d) T. Okino, S. Nakamura, T. Furukawa, Y. Takemoto, Enantioselective aza-Henry reaction catalyzed by a bifunctional organocatalyst, Org. Lett. 6 (2004) 625-627; (e) Y. Hoashi, T. Yabuta, P. Yuan, H. Miyabe, Y. Takemoto, Enantioselective tandem Michael reaction to nitroalkene catalyzed by bifunctional thiourea: total synthesis of ( )-epibatidine, Tetrahedron 62 (2006) 365-374; (f) H.J. Yang, F.J. Xiong, J. Li, F.E. Chen, A family of novel bifunctional organocatalysts: highly enantioselective alcoholysis of meso cyclic anhydrides and its application for synthesis of the key intermediate of P2X7 receptor antagonists, Chin. Chem. Lett. 24 (2013) 553-558. |
| [10] | (a) E. Maalej, F. Chabchoub, A. Samadi, et al., Synthesis, biological assessment and molecular modeling of 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[ 2,3-b]quinolin-13-amines, Bioorg. Med. Chem. Lett. 21 (2011) 2384-2388; (b) N.P. Selvam, T.H. Babu, P.T. Perumal, A simple and convenient approach to the Friedländer synthesis of pyrano[2,3-b]pyridines, Tetrahedron 65 (2009) 8524-8530; (c) J.R. Li, L.J. Zhang, D.X. Shi, et al., A new conversion of Friedla¨ndler reaction and the structure of its products, Synlett (2008) 233-236. |
| [11] | X-ray crystal: Triclinic, space group: P-1, Final R indices [I>2sigma(I)]: R1 = 0.0537, wR2 = 0.1763; unit cell dimensions: a = 7.6593(12) Å, b = 13.191(2) Å, c = 13.678 (2)Å, α = 67.787(2)8, β = 82.977(3)8, γ = 86.498(2)8; volume: 1269.7(3) Å3; crystal size: 0.39 mm×0.30 mm×0.28 mm; T = 173(2) K; Z, calculated density: 2, 1.416 mg/m3; reflections collected/unique: 9139/5448[R(int) = 0.0219]; date/restraints/parameters: 5448/0/316. X-ray analysis of single crystal of 5 was deposited at Cambridge crystallographic data center with CCDC 862430. |
| [12] | (a) K. Faber, H. Stueckler, T. Kappe, Non-steroidal antiinflammatory agents. 1. Synthesis of 4-hydroxy-2-oxo-1, 2-dihydroquinolin-3-yl alkanoic acids by the Wittig reaction of quinisatines, J. Heterocycl. Chem. 21 (1984) 1177-1181; (b) J.V. Johnson, B.S. Rauckman, P.D. Beccanari, B. Roth, 2,4-Diamino-5-benzylpyrimidines and analogs as antibacterial agents. 12. 1,2-Dihydroquinolylmethyl analogs with high activity and specificity for bacterial dihydrofolate reductase, J. Med. Chem. 32 (1989) 1942-1949; (c) N. Yamada, S. Kadowaki, K. Takahashi, K. Umeza, MY-1250, a major metabolite of the anti-allergic drug repirinast, induces phosphorylation of a 78-kDa protein in rat mast cells, Biochem. Pharmacol. 44 (1992) 1211-1213. |
| [13] | (a) G. Kolokythas, N. Pouli, P. Marakos, H. Pratsinis, D. Kletsas, Synthesis and antiproliferative activity of some new azapyranoxanthenone amino derivatives, Eur. J. Med. Chem. 41 (2006) 71-79; (b) M.A. Azuine, H. Tokuda, J. Takayasu, et al., Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradecanoylphorbol- 13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models, Pharmacol. Res. 49 (2004) 161-169; (c) S.K. Srivastava, R.P. Tripathi, R. Ramachandran, NAD+-dependent DNA ligase (rv3014c) from mycobacterium tuberculosis: crystal structure of the adenylation domain and identification of novel inhibitors, J. Biol. Chem. 280 (2005) 30273- 30281; (d) H. Brotz-Oesterhelt, I. Knezevic, S. Bartel, et al., Specific and potent inhibition of NAD+-dependent DNA ligase by pyridochromanones, J. Biol. Chem. 278 (2003) 39435-39442. |