b Jiangsu Marine Resources Development Research Institute, Lianyungang 222005, China
Possessing both the structures of amino acid and sugar,glycosyl amino acids have been extensively studied in recent years [1, 2, 3]. They are important building blocks for synthesis of glycoproteins and have potential utilities in medicinal chemistry [4, 5, 6, 7, 8]. Because of the limited accessibility of well-defined glycoproteins from natural sources,recent efforts have been focused on the chemical synthesis of glycosyl amino acids where the amino acid side chains are connected to the sugar unit via linkers such as O-linker, C-linker,S-linker and N-linker (Fig. 1) [9, 10, 11, 12].

|
Download:
|
| Fig. 1.Glycosyl amino acids. | |
Among the glycosyl amino acids,N-glycosyl a-amino acids have gained great attention (Fig. 1) because they not only have significant biological activity [13, 14, 15] but also can be used as ligands in coordination chemistry [16]. The current method to prepare N-glycosyl a-amino acids is to condense sugar with amino acids,where the amino acids are usually natural [13, 14, 15, 16]. However, unnatural N-glycosyl a-amino acids,which are from unnatural amino acids,have rarely been studied due to their synthetic difficulties. Recently,we have been interested in carbohydrate chemistry and developed several general protocols to synthesize the derivatives of D-glucosamine [17, 18]. Herein,we report an efficient,high-yielding synthesis of unnatural N-glycosyl a-amino acids (1) through Petasis reaction (Fig. 2) [19, 20].
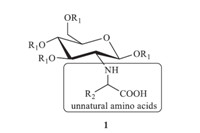
|
Download:
|
| Fig. 2.Unnatural N-glycosyl a-amino acids. | |
To the best of our knowledge,this is the first time that Petasis reaction has been employed to synthesize biologically important N-glycosyl a-amino acids. Accessibility of the reagents and the mild reaction conditions make the method highly practical [21- 23]. More importantly,owing to the easy functionalization of C55C double bonds,this protocol can provide various glycosyl amino acids for glycoproteins synthesis.
All chemicals were obtained from commercial sources and used without further purification. Flash column chromatography was performed on silica 230-400 mesh. IR spectra were recorded on a Bruker Tensor 27 spectrometer with KBr pellets. 1H NMR spectra were recorded on a Bruker Advance 400 spectrometer at ambient temperature in CDCl3. Chemical shifts were reported in ppm relative to TMS. HRMS analysis was performed on a Mariner ESITOF system.
Synthesis of N-glycosyl a-amino acids: A 50 mL round-bottom flask containing a magnetic stirring bar was charged with D-glucosamine hydrochloride 2a or its derivative 2b (1.0 mmol, 1 equiv.),glyoxylic acid hydrate (1.0 mmol,1 equiv.),and alkenyl boronic acid (1.0 mmol,1 equiv.). CH2Cl2 (5.0 mL) and triethylamine (1.0 mmol,1 equiv.) were injected,and the suspension was stirred for 24 h at room temperature. The resulting mixture was filtered through a pad of silica gel with the help of CH2Cl2 (30 mL). The filtrate was concentrated,and the residue was purified by column chromatography (silica gel,EtOAc-PE) to afford the product 1 (see Supporting information).
We commenced our study with styryl boronic acid,glyoxylic acid,and D-glucosamine hydrochloride (2a) as model substrates. Petasis reaction was carried out at room temperature and triethylamine was added to release the free amine of D-glucosamine. Different solvents were examined but we could not obtain the designed N-glycosyl a-amino acids 1 from D-glucosamine (Table 1,entries 1-5). We speculated that the hydroxyl groups, especially the 1-hydroxyl,influenced the reaction. Then,we turned to the substrate derived from O-protected-D-glucosamine.
| Table 1 Optimizing the Petasis reaction condition.a |
It is well-known that acetyl is a versatile group for protecting hydroxyl groups in carbohydrate chemistry and can be removed under mild conditions [24]. If 1,3,4,6-tetra-O-acetyl-b-D-glucosamine hydrochloride (2b) was used as surrogate of D-glucosamine hydrochloride (2a),it would probably easily participate in the Petasis reaction to produce N-glycosyl a-amino acids. This hypothesis prompted us to investigate 1,3,4,6-tetra-O-acetyl-b- D-glucosamine hydrochloride as a surrogate of D-glucosamine. Gratifyingly,the designed product (1) was obtained with 15% - 20% yields in protic solvents CH3OH and CH3CH2OH (entries 6 and 7). Then,we evaluated the polar solvents,and the yield was increased to 85%when CH2Cl2 was used as solvent (entries 8-10). Nevertheless,less polar solvents were not suitable for the transformation,and only 11% yield was obtained with toluene as the solvent (entry 11). Therefore,our optimal protocol for synthesis of N-glycosyl a-amino acids was as follows: 1,3,4,6-tetra-O-acetyl-b-D-glucosamine hydrochloride (2b) as the surrogate of D-glucosamine hydrochloride,CH2Cl2 as the solvent at room temperature.
Subsequently,we tested whether the same system can be applied to the coupling of 1,3,4,6-tetra-O-acetyl-b-D-glucosamine hydrochloride with various boronic acids and carboxyl acids. It was found that other substituted alkenyl boronic acids could also be smoothly converted to the desired products. For instance,when pentenyl boronic acid was used,the designed N-glycosyl a-amino acid 1b was obtained with high isolated yield (up to 88%,Scheme 1).

|
Download:
|
| Scheme 1.Synthesis of N-glycosyl a-amino acid 1b. | |
However,other carbonyl acids are minimally conducive to the reaction. For example,even at 40 8C,only 9% yield was obtained when keto acid was introduced (Scheme 2).

|
Download:
|
| Scheme 2.Synthesis of N-glycosyl a-amino acid 1c. | |
Then,we investigated the stereoselectivities of N-glycosyl a-amino acids through the 1H NMR of the products 1a and 1b (see figures in Supporting information). Since the amine of 1,3,4,6- tetra-O-acetyl-b-D-glucosamine hydrochloride (2b) is chiral,we expected the chirality could be introduced to the products of Nglycosyl a-amino acids. Nevertheless,styryl boronic acid gave 1a with only 17% de,and the use of pentenyl boronic acid was much less selective (1b,11% de). Now,further studies of the stereochemistry of this process are currently underway in our lab.
In summary,we have developed a convenient and mild protocol for the synthesis of unnatural N-glycosyl a-amino acids. 1,3,4,6- Tetra-O-actyl-b-D-glucosamine hydrochloride (2b) was used as the surrogate of D-glucosamine hydrochloride,and alkenyl boronic acid and glyoxylic acid were used to achieve Petasis reaction. The efficiency of this procedure has been demonstrated by synthesizing derivatives of 2-(N-glycosyl)aminobut-3-enoic acids. Given the fact that glycosyl amino acids play an important role in glycobiology research and medicinal chemistry,we anticipate that the method described in the present report will find applications in a number of fields,such as biomedical and pharmaceutical researches.
We are grateful to the Natural Science Foundation of Jiangsu Province (No. BK20130404),the Scientific and Technological Research Project of Lianyungang (No. CG1303),and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.01.035.
| [1] | S.A.W. Gruner, E. Locardi, E. Lohof, H. Kessler, Carbohydrate-based mimetics in drug design: sugar amino acids and carbohydrate scaffolds, Chem. Rev. 102 (2002) 491-514. |
| [2] | P.M. St. Hilaire, T.L. Lowary, M. Meldal, K. Bock, Oligosaccharide mimetics obtained by novel, rapid screening of carboxylic acid encoded glycopeptide libraries, J. Am. Chem. Soc. 120 (1998) 13312-13320. |
| [3] | L. Jobron, G. Hummel, Solid-phase synthesis of unprotected N-glycopeptide building blocks for SPOT synthesis of N-linked glycopeptides, Angew. Chem. Int. Ed. 39 (2000) 1621-1624. |
| [4] | L. Liu, C.S. Bennett, C.H. Wong, Advances in glycoprotein synthesis, Chem. Commun. (2006) 21-33. |
| [5] | D.P. Gamblin, E.M. Scanlan, B.G. Davis, Glycoprotein synthesis: an update, Chem. Rev. 109 (2009) 131-163. |
| [6] | G.M. Fang, Y.M. Li, F. Shen, et al., Protein chemical synthesis by ligation of peptide hydrazides, Angew. Chem. Int. Ed. 50 (2011) 7645-7649. |
| [7] | Y. Hajihara, M. Izumi, K. Hirano, et al., Elucidating the function of complex-type oligosaccharides by use of chemical synthesis of homogeneous glycoproteins, Isr. J. Chem. 51 (2011) 917-929. |
| [8] | J.S. Zheng, H.N. Chang, F.L. Wang, L. Liu, Fmoc synthesis of peptide thioesters without post-chain-assembly manipulation, J. Am. Chem. Soc. 133 (2011) 11080-11083. |
| [9] | V.L. Campo, I. Carvalho, S. Allman, B.G. Davis, R.A. Field, Chemical and chemoenzymatic synthesis of glycosyl-amino acids and glycopeptides related to Trypanosoma cruzi mucins, Org. Biomol. Chem. 5 (2007) 2645-2657. |
| [10] | A. Nuzzi, A. Massi, A. Dondoni, General synthesis of C-glycosyl amino acids via proline-catalyzed direct electrophilic a-amination of C-glycosylalkyl aldehydes, Org. Lett. 10 (2008) 4485-4488. |
| [11] | S.B. Cohen, R.L. Halcomb, Application of serine- and threonine-derived cyclic sulfamidates for the preparation of S-linked glycosyl amino acids in solution- and solid-phase peptide synthesis, J. Am. Chem. Soc. 124 (2002) 2534-2543. |
| [12] | A.L. Handlon, B. Fraser-Reid, A convergent strategy for the critical .beta.-linked chitobiosyl-N-glycopeptide core, J. Am. Chem. Soc. 115 (1993) 3796-3797. |
| [13] | G. Geisberger, E.B. Gyenge, D. Hinger, et al., Chitosan-thioglycolic acid as a versatile antimicrobial agent, Biomacromolecules 14 (2013) 1010-1017. |
| [14] | S.M. Srinivas, N.V. Harohally, Improved synthesis of lysine- and argininederived amadori and Heyns products and in vitro measurement of their angiotensin I-converting enzyme inhibitory activity, J. Agric. Food Chem. 60 (2012) 1522-1527. |
| [15] | V.V. Mossine, C.L. Barnes, G.V. Glinsky, M.S. Feather, Molecular and crystal structure of N-(2-deoxy-D-aldohexos-2-yl)-glycines (Heyns compounds), Carbohydr. Res. 284 (1996) 11-24. |
| [16] | K. Stefan, B. Wolfgang, Metal complexes of biologically important ligands. CXXXI. Pentamethylcyclopentadienyl halfsandwich complexes of rhodium and iridium with glycosyl-alpha-iminocarboxylates as chelate ligands, Z. Naturforsch. B 56 (2001) 62-68. |
| [17] | C.Z. Tao, F. Liu, W.W. Liu, et al., Synthesis of N-aryl-D-glucosamines through copper-catalyzed C-N coupling, Tetrahedron Lett. 53 (2012) 7093-7096. |
| [18] | C.Z. Tao, F. Liu, B. Xu, et al., Copper-catalyzed synthesis of N-aryl-D-glucosamines from arylboronic acids, J. Carbohydr. Chem. 32 (2013) 411-423. |
| [19] | Z.Y. Hong, L. Liu, C.C. Hsu, C.H. Wong, Three-step synthesis of sialic acids and derivatives, Angew. Chem. Int. Ed. 45 (2006) 7417-7421. |
| [20] | Z.Y. Hong, L. Liu, M. Sugiyama, Y. Fu, C.H. Wong, Concise synthesis of iminocyclitols via Petasis-type aminocyclization, J. Am. Chem. Soc. 131 (2009) 8352-8353. |
| [21] | H.J. Xu, Y.Q. Zhao, T. Feng, Y.S. Feng, Chan-Lam-type S-arylation of thiols with boronic acids at room temperature, J. Org. Chem. 77 (2012) 2878-2884. |
| [22] | J.J. Dai, J.H. Liu, D.F. Luo, L. Liu, Pd-catalysed decarboxylative Suzuki reactions and orthogonal Cu-based O-arylation of aromatic carboxylic acids, Chem. Commun. 47 (2011) 677-679. |
| [23] | C.T. Yang, Z.Q. Zhang, Y.C. Liu, L. Liu, Copper-catalyzed cross-coupling reaction of organoboron compounds with primary alkyl halides and pseudohalides, Angew. Chem. Int. Ed. 50 (2011) 3904-3907. |
| [24] | P.G.M. Wuts, T.W. Greene (Eds.), Greene's Protective Groups in Organic Synthesis, 4th ed., John Wiley & Sons, New York, 2007. |





