b Department of Food and Environment Engineering, Heilongjiang East University, Harbin 150086, China
Recently,considerable interest has been devoted to the growth of crystalline inorganic structures with specific shapes and hierarchies at micrometer scale for their applications in designing new functional materials and devices in various fields. Especially, thematerialswithshapeslikeflower,treeandleaveattractedmore attention for bionic design [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Such patterned materials are currently manufactured in a top-down manner to produce the designed crystalline structures. The process generally involves the ‘‘writing’’ of the target pattern using lithographic techniques and various etching procedures. Nevertheless the alternative,bottom- up crystallization route is more attractive using the principles borrowed from nature. This nonlithographic synthesis of func- tional bionic structures is an important task for next-generation nanofabrication. Among the bottom-up methods,the template- induced growth is more attractive for the controlling of the crystal assembly [14, 15, 16]. For example,Colorado et al. have prepared SFS hollow hexagonal nuts by in situ growing composite of SFS and single-walled carbon nanotubes (SWCTs) in a dissolution and regrowth process [17]. In their experiment,the additive,SWCTs play an important role. In the present communication,we prepared trumpet-like sodium hexafluorosilicate (SFS) flowers via a similar dissolution and regrowth process. But the difference is that in our experimental process a template was used and gas was generated.
2. ExperimentalSynthesis of silica spheres: Seed suspensions were prepared according to the published procedures [18]. The growth of 450 nm silica spheres was controlled as the following procedure: 20 mL of seed suspensions and 7.0 g of ammonium hydroxide were diluted to 100 mL by distilled water in a three-neck flask at 40 8C under stirring (200 rpm). Then 25.0 g of TEOS in 80 mL of anhydrous ethanol and 5.7 g of ammonium hydroxide in 80 mL of anhydrous ethanol were added dropwise synchronously in 3 days using two syringe pumps.
Preparation of CF: Firstly,the 450 nm silica opal was deposited on a glass substrate by the vertical deposition method [19]. Secondly the opal composed of 450 nm silica spheres was sintered at 600 8C for 1 h and kept under heat preservation for 1 h,annealed to room temperature spontaneously. Finally,an appropriate amount of 20 wt% polystyrene toluene solution was infiltrated into the air voids of the silica opal template,and the mixture was dried under vacuum at 80 8C for 2 h,then cooled to 20 8C and the composite film (CF) of polystyrene with silica spheres was obtained [20, 21].
Trumpet-like flowers growth process: The CF was soaked in a 20 wt% hydrofluoricacidaqueoussolutionatabout15 8C. Theglass substrate was dissolved layer by layer and slides down into the hydrofluoric acid solution. When dissolved to the last layer,which sintered with the silica spheres,the mixture was added to a fresh 4 wt% hydrofluoric acid aqueous solution at about 15 8C. Then the crystals on OPPF were washed with distilled water and kept for SEM and XRD detection.
3. Results and discussionThe morphology of the SFS crystalline can be observed by SEM, presented in Fig. 1. As can be seen,the whole structure looks like a 3D mini-size garden. The size of flowers is about micrometer scale and the shape is hexagonal trumpet-like. To determine why the flowers exhibit a hexagonal shape,we analyzed the structures of the sample using X-ray diffraction (XRD) (Fig. 2). The patterns of the flowers fit well with the presence of malladrite (sodium hexafluorosilicate,NaSiF6,Joint Committee on Power Diffraction Standards [JCPDS] Card No. 33-1280)

|
Download:
|
| Fig. 1.SEM image of SFS flowers on OPPF surface. The inset shows one flower with high-magnification. | |

|
Download:
|
| Fig. 2.X-ray diffraction patterns of the SFS flowers. | |
In light of the fact that SFS exhibits hexagonal crystals when crystallized [17],we propose that HF may dissolve away sodium silicate of the glass substrate and regrow it on the surface of the template. When the CF was soaked in a 20 wt% HF aqueous solution,the silica spheres were dissolved and SiF4formed. This process involves the formation of SFS (Eq. (1)) and the equilibrium between silica and HF (Eq. (2)).
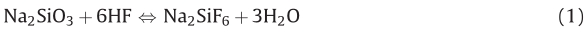

To clarify what makes SFS crystallize on the OPPF surface,we took an OPPF sheet suspending in a solution where the HF etched glass substrate. Under the same conditions,there were no crystals on the OPPF surface,indicating that the clean OPPF cannot induce crystallization since the polystyrene is hydrophobic. So,the silica spheres’ dissolution process must have induced the SFS growth on the OPPF surface,and both processes occurred simultaneously.
To understand why the crystalline structure is trumpet-like but not hex nuts orhollowhex nuts,wefurtherinvestigate thisunusual dissolution/regrowth process by monitoring the etching process using SEM (shown in Fig. 3). Indeed,this analysis reveals a series of stagesthatallowustorationalizetheformationofthefinaltrumpet- like flowers. The initial stage of deposition is indicated by the appearance of raised circular bumps on the surface of the OPPF surface (Fig.3a).Wededucethatthesebumpsformasaresultofthe process of the glass substrate’s dissolution. In succession,the original silica spheres and sodium silicate exposed on the surface of theCFareetched,andtheyconverttoSiF4andSFS,respectively.The SFS continues depositing onto the surface of bumps. When SiF4 blowsupward,newbumpswithporesappearedonthetop(Fig.3b). The SiF4was derived from SiO2opal as shown in Eq. (2). Then the crystallization process continues (Fig. 3c). At this point,the SFS still depositsontothesurfaceofbumps.Duetotheexistenceofthepores, the crystals are hollow. It is just this gas that induces the expansion ofthecrystals’toprims,justlikethecaseintrumpetflowers.Finally, a minimized garden forms (Fig. 3d).

|
Download:
|
| Fig. 3.SEM images showing the stages of the trumpet-like flowers growth on an ordered porous polystyrene film. (a) Bumps dense packed,(b) bumps with pores occurred,(c) more pumps with pores grown up and (d) final trumpet-like flowers. | |
We also obtained hollow tubes as the literature indicated [17]. From Fig. 4a,one can observe the shape of the bumps was different from those in Fig. 3b and c. This illustrated that it was the gas that enlarged the span of the top. Obviously,to produce SFS hollow nuts,the preliminary deposition with pores of SFS is essential.Fig. 4a illustrated this hypothesis. As Fig. 4b shows,if SFS has not deposited on the film with pores,the crystals were not hollow tubes,but hexagonal-shaped crystals as the literature reported [22]. To generate SFS hollow nuts,the preliminary deposition with holes of SFS is essential.

|
Download:
|
| Fig. 4.SEM images of (a) tubes grown on porous polystyrene film,the inset shows one tube with high-magnification and (b) hexagonal-shaped crystals on the part of the polystyrene. | |
In conclusion,we have fabricated SFS flowers by a synchronous dissolution/regrowth method. Their formation process can be divided into several steps: first,the dissolution of the silica spheres induced the crystallization of SFS onto the OPPF; second,some pores emerged on the closely packed bumps when being blown by the SiF4gas; third,when the crystal was blown by continuous gas from the pores,the span of the top became larger than that of the bottom. In our fabrication procedure,the SiF4 gas played an important role in impelling the crystals to grow into trumpet-like flowers.
AcknowledgmentsThe present study has been supported by the National Natural Science Foundation of China (Nos. 51273056, 21202091, 5121010502,21074031),Postdoctoral ScienceFoundationProjects of China (No. 2013M531008) and Heilongjiang Provincial Department of Education (No. 12521398).
| [1] | L.S. Zhong, J.S. Hu, H.P. Liang, et al., Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment, Adv. Mater. 18 (2008) 2426-2431. |
| [2] | J.M. Wu, B. Huang, M. Wang, A. Osaka, Titania nanoflowers with high photocatalytic activity, J. Am. Ceram. Soc. 89 (2006) 2660-2663. |
| [3] | S.O. Cho, E.J. Lee, H.M. Lee, J.G. Kim, Y.J. Kim, Controlled synthesis of abundantly branched, hierarchical nanotrees by electron irradiation of polymers, Adv. Mater. 18 (2006) 60-65. |
| [4] | J. Han, G. Song, R. Guo, Nanostructure-based leaf-like polyaniline in the presence of an amphiphilic triblock copolymer, Adv. Mater. 19 (2007) 2993-2999. |
| [5] | S. Wang, L. Feng, L. Jiang, One-step solution-immersion process for the fabrication of stable bionic superhydrophobic surfaces, Adv. Mater. 18 (2006) 767-770. |
| [6] | L.N. Jin, Q. Liu, W.Y. Sun, Room temperature solution-phase synthesis of flowerlike nanostructures of [Ni3(BTC)2 12H2O] and their conversion to porous NiO, Chin. Chem. Lett. 24 (2013) 663-667. |
| [7] | Z.P. Zhang, X.Q. Shao, H.D. Yu, Y.B. Wang, M.Y. Han, Morphosynthesis and ornamentation of 3D dendritic nanoarchitectures, Chem. Mater. 17 (2005) 332-336. |
| [8] | C. O'Dwyer, D. Navas, V. Lavayen, et al., Nano-urchin: the formation and structure of high-density spherical clusters of vanadium oxide nanotubes, Chem. Mater. 18 (2006) 3016-3022. |
| [9] | L.P. Xu, Y.S. Ding, C.H. Chen, et al., 3D flowerliker-nickel hydroxide with enhanced electrochemical activity synthesized by microwave-assisted hydrothermal method, Chem. Mater. 20 (2008) 308-316. |
| [10] | F.H. Zhao, X.Y. Li, J.G. Zheng, et al., ZnO pine-nanotree arrays grown from facile metal chemical corrosion and oxidation, Chem. Mater. 20 (2008) 1197-1199. |
| [11] | J.M. Wu, B. Qi, Low-temperature growth of monolayer rutile TiO2 nanorod films, J. Am. Ceram. Soc. 90 (2007) 657-660. |
| [12] | Y.S. Sun, A.L. Li, F.J. Xu, D. Qiu, A low-temperature sol-gel route for the synthesis of bioactive calcium silicates, Chin. Chem. Lett. 24 (2013) 170-172. |
| [13] | J. Aizenberg, Crystallization in patterns: a bio-inspired approach, Adv. Mater. 16 (2004) 1295-1302. |
| [14] | M. Goodman, Y.B. Feng, G. Melacini, J.P. Taulane, A template-induced incipient collagen-like triple-helical structure, J. Am. Chem. Soc. 118 (1996) 5156-5157. |
| [15] | C.G. Oh, Y.Y. Baek, S.K. Ihm, Synthesis of skeletal-structured bioporous silicate powders through microcolloidal crystal templating, Adv. Mater. 17 (2005) 270-273. |
| [16] | D.K. Yi, D.Y. Kim, Novel approach to the fabrication of macroporous polymers and their use as a template for crystalline titania nanorings, Nano Lett. 3 (2003) 207-211. |
| [17] | R. Colorado Jr., M.E. Diosomito, A.R. Barron, In-situ fabrication of freestanding single-walled carbon nanotube-silicate composite hex nuts, Adv. Mater. 17 (2005) 1634-1637. |
| [18] | H. Giesche, Synthesis of monodispersed silica powders. Ⅱ. Controlled growth reaction and continuous production process, J. Eur. Ceram. Soc. 14 (1994) 205-214. |
| [19] | P. Jiang, J.F. Bertone, K.S. Hwang, V.L. Colvin, Single-crystal colloidal multilayers of controlled thickness, Chem. Mater. 11 (1999) 2132-2140. |
| [20] | Z.Y. Xie, L.G. Sun, G.Z. Han, Z.Z. Gu, Optical switching of a birefringent photonic crystal, Adv. Mater. 20 (2008) 1-4. |
| [21] | Y.J. Zhao, X.W. Zhao, J. Hu, et al., Encoded porous beads for label-free multiplex detection of tumor markers, Adv. Mater. 21 (2009) 569-572. |
| [22] | R. Chandrasekhar, Influence of magnetic field on sodium hexafluorosilicate synthesis, J. Cryst. Growth 216 (2000) 407-412. |




