b College of Science, Jiangxi Agricultural University, Nanchang 330045, China
1. Introduction
Quercetin (3,30,40,5,7-penta hydroxyl flavones),one of the most abundant natural flavonoids,has been widely distributed in various vegetables and fruits,especially in traditional Chinese herbs. Its average human daily intake is estimated to be 16-25 mg per person. Over the past years,quercetin has drawn much attention because of its various beneficial effects on human health, including anti-cancer,anti-inflammatory,anti-tumor,anti-ulcer, anti-allergy,anti-viral,and anti-oxidant effects [1, 2],and quercetin also can protect human DNA from oxidative attack in vitro [3]. Hence,it is extremely important to determine and study it.
Several techniques have been utilized in the determination of quercetin,for example,HPLC-UV [4],spectrophotometry [5],liquid chromatography with mass spectrometry [6],and solid phase extraction [7]. These methods are highly sensitive and effective, but often require some complicated and time consuming sample pretreatment. Due to the advantages of time-saving,simple operation,sensitivity,low-cost,and on-field detection,some electrochemical methods have been proposed for the electrochemical study of quercetin. Several materials have been used to fabricate the modified electrode,such as carbon nanotubes [3,8- 10],copper microparticles [11],and graphene nanosheets [12]. However,to date,the application of conductive polymer with metal nanoparticles for the electroanalytical detection of quercetin has not been reported.
operation,sensitivity,low-cost,and on-field detection,some electrochemical methods have been proposed for the electrochemical study of quercetin. Several materials have been used to fabricate the modified electrode,such as carbon nanotubes [3,8- 10],copper microparticles [11],and graphene nanosheets [12]. However,to date,the application of conductive polymer with metal nanoparticles for the electroanalytical detection of quercetin has not been reported.attention for the application of chemo/bio-sensors materials because of the high conductivity,good stability,friendly biocompatibility, low toxicity,and relatively low band gap [20, 21, 22, 23, 24]. Hydroxymethylated-3,4-ethylenedioxylthiophene (EDOT-MeOH), a polar derivative of EDOT,exhibited better water-solubility and a lower onset oxidation potential compared with EDOT monomer [25, 26, 27, 28, 29]. In addition,our group has reported a new and efficient synthetic route to obtain EDOT-MeOH monomer and the fabricated PEDOT-MeOH chemo/bio-sensors were successfully used in the electrochemical determination of the anticancer herbal drug, shikonin [27],and VC in commercial fruit juice [27, 28]. Therefore, it is very meaningful to construct an electrochemical sensor for the determination of quercetin by incorporating the merits of EDOTMeOH and PtNPs.
In this contribution,in view of the merits of conducting polymer PEDOT-MeOH and metal nanoparticles PtNPs,a simple and sensitive PtNPs/PEDOT-MeOH nanocomposite modified glassy carbon electrode (GCE) was constructed for the electrochemical determination of quercetin. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) results showed that the PtNPs were inserted into the PEDOT layer and formed a porous 3D structure,which exhibited large surface area and excellent electrocatalytic activity for the oxidation of quercetin. Moreover,the electrochemical properties of the PtNPs/PEDOTMeOH/ GCE modified electrode for the voltammetric detection of quercetin were studied.
2. Experimental 2.1. Chemicals
3,4-Dibromothiophene (EDOT) (98%,Shanghai Bangcheng Chemical Co.,Ltd.),Quercetin (>98%) and platinum powder (99.99%) were obtained from Aladdin Reagent Co.,Ltd. All other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. with analytical grade and were used as received without any further purification. All solutions were prepared using doubledistilled deionized water.
2.2. Apparatus
Electrochemical measurements were performed on a CHI660B electrochemicalworkstation(Chenhua InstrumentCompany,Shanghai, China) with a conventional electrochemical cell containing a three-electrode system. Themodified electrode or bare glassy carbon electrode (GCE) (F = 3mm) was served as a working electrode,a platinum wire (F = 1mm) was used as a counter electrode,and a saturated calomel electrode (SCE) as the reference electrode. The addition of samples was performed with a microsyringe (Shanghai Gaoge Industry & Trade Co.,Ltd.,China). The pH values of PBS were measured with a PHB-5 portable pH meter (Hangzhou Qiwei Instrument,China). SEMmeasurementswere taken with a JEOL JSM- 6701F scanning electron microscope (Tokyo,Japan).
2.3. Preparation of modified electrode
Prior to modification,the working electrode bare GCE was
carefully polished with 0.05 mm alumina slurry until a mirrorshine
surface was obtained,followed by successively sonicating in
doubly distilled deionized water and ethanol,and then dried in air.
The PEDOT-MeOH/GCE modified electrode was prepared facilely
by one-step electropolymerization of EDOT-MeOH (10 mmol L-1)
in 0.1 mol L-1 LiClO4. The deposition potential was 1.1 vs. SCE and
the deposition time is 70 s. After drying at room temperature under
a clean environment,the PEDOT-MeOH/GCE was electrodeposited
in 5 mmol L-1 HPtCl6 solution by cyclic voltammetry (CV) at the
scan rate of 100 mV s
2.4. Experimental measurements
Five milliliters of 0.1 mol L-1 PBS (pH 7.0) with a specific
amount of quercetin solution was transferred into the sealed
electrochemical cell by microsyringe. The voltammetric detection
of quercetin was studied by differential pulse voltammetry (DPV).
The DPV conditions were as follows: potential increase,0.004 V;
amplitude,0.05 V; pulse width,0.05 s; and pulse interval,0.2 s.
Prior to each experiment,all solutions were deoxygenated by
purging with nitrogen for 10 min. The fabrication process of the
modified electrode and the working mechanism for the electrochemical
analysis of quercetin are shown in Scheme 1.
3. Results and discussion
3.1. Characterization of modified electrodes
The surface morphology of the PEDOT-MeOH film and the
PtNPs/PEDOT-MeOH composite deposited on the indium-tin oxide
(ITO) transparent electrode was investigated by SEM. As shown in
Fig. 1A,The PEDOT-MeOH film obtained from the aqueous solution
was rough and compact structure,while the morphology of PtNPs/
PEDOT-MeOH film exhibited a three-dimensional (3D) morphological
structure (Fig. 1B). The 3D structure of PtNPs/PEDOT-MeOH
modified electrode could produce a large surface area structure,
which is beneficial to maintaining large electroactive area on the
electrode surface. Moreover,the 3D structure may give a high
sensitivity for the detection of analytes. In addition,energy
dispersive spectrum (EDS) analysis was performed to further
confirm the components of the PtNPs/PEDOT-MeOH film shown in
Fig. 1C. The presence of C,S,O,and Pt come from the component
elements of the PEDOT-MeOH-PtNPs composite. Other elements
mainly originated from electrolysis solution and ITO. The content
of PtNPs is shown in Table 1. All the results confirm the presence of
Pt on PEDOT-MeOH-PtNPs composite.
Electrochemical impedance spectroscopy (EIS) was performed
at the potential of 0.23 V and the frequency range from 10 kHz to
100 mHz,using 5 mmol L-1 [Fe(CN)6]3-/4- redox couple (1:1) with
0.1 mol L-1 KCl,as supporting electrolyte. The value of the
modified electrode was estimated by the semicircle diameter.
Fig. 2A illustrates the EIS of bare GCE (a),PEDOT-MeOH/GCE (b),
and PtNPs/PEDOT-MeOH/GCE (c). Obviously,the bare GCE
exhibited a semicircle portion and the value of electron-transfer
resistance (Rct) was estimated to be 200 V. However,compared to
the bare GCE,instead of the semicircle part,the PEDOT-MeOH/GCE
and PtNPs/PEDOT-MeOH/GCE were linear curves,implying the
PEDOT-MeOH films had a good capacitance behavior [29].
Cyclic voltammetric experiments were further carried out in
5 mmol L-1 [Fe(CN)6]3-/4- containing 0.1 mol L-1 KCl,shown in
Fig. 2B. A pair of well-defined anodic and cathodic peaks was
observed on all modified electrodes. The potential peak separation
(DEp) between the anodic and cathodic potential peaks PEDOTMeOH/
GCE (96 mV,curve b) and PtNPs/PEDOT-MeOH/GCE
(92 mV,curve c) were smaller than that at bare GCE (116 mV,
curve a),indicating that PEDOT-MeOH films were electropolymerized
on the surface of bare GCE and PtNPs were electrodeposited on
the surface of PEDOT-MeOH/GCE,and also implying that the
reversibility of electrochemical reaction at PEDOT-MeOH films
modified electrodes were improved. Meanwhile,the response
currents were higher than bare GCE,which was attributed to the
good conductivity of PEDOT-MeOH films.
3.2. Electrochemical behavior of quercetin
Fig. 3 shows cyclic voltammograms (CVs) of 50 mmol L-1
quercetin in 0.1 mol L-1 PBS (pH 7.0) at different electrodes: bare
GCE (a),PEDOT-MeOH/GCE (b),and PtNPs/PEDOT-MeOH/GCE (c).
It can be seen that besides a pair of well-defined redox peaks at
PtNPs/PEDOT-MeOH/GCE,quercetin showed an irreversible cathodic
peak (II). The oxidation of the catechol moiety,30,40-
dihydroxyl groups at ring A,occurs first at very low positive
potential corresponding to peak I [8, 30]. The further scan to
positive potential displayed an additional oxidation peak,known
as the oxidation of other hydroxyl groups (Scheme 2). As also can
be seen at the bare GCE,quercetin was reversibly oxidized with an
anodic peak potential at around 0.19 V,while the anodic peak
potential of quercetin at PtNPs/PEDOT-MeOH/GCE (0.13 V) shifted
more negatively than that at PEDOT-MeOH/GCE (0.16 V) and bare
GCE (0.19 V),suggesting that PtNPs could reduce the overpotential
of quercetin and accelerate the electron transfer rate,which due to
their subtle electronic properties [14]. Furthermore,the redox peak
currents of quercetin on PtNPs/PEDOT-MeOH/GCE and PEDOTMeOH/
GCE were stronger than that on bare GCE. These results
mean that both PEDOT-MeOH and PtNPs had an electrocatalytic
activity toward quercetin,and the synergic effect of PEDOT-MeOH
and PtNPs as co-catalysts made the PtNPs/PEDOT-MeOH/GCE
show higher electrocatalytic activity than PEDOT-MeOH/GCE.
PtNPs/PEDOT-MeOH films promoted the electrochemical reaction
of quercetin more efficiently.
3.3. Optimization of conditions
To achieve a highly sensitive detection of quercetin,experimental
parameters: preconcentration time and pH value have been
optimized.
The effect of pH value on the determination of quercetin at
PtNPs/PEDOT-MeOH/GCE over the range of pH 3.0-9.0 was
investigated by CVs. As shown in Fig. 4A (solid line),the anodic
peak currents of quercetin at PtNPs/PEDOT-MeOH/GCE increased
gradually with the increasing pH value until it attained the
maximum at pH 7.0. Thus,pH 7.0 was chosen in the following
experiments as the best pH condition. The relationship between
anodic peak potentials (Ep) and pH value was also demonstrated in
Fig. 4A (dotted line). Ep shifted negatively with the increasing of pH
values and the equation was Ep (V) = -0.06581 pH + 0.6161
(R2 = 0.99898). The slope (65.8 mV pH-1) of the equation is
approximately close to the theoretical value of 58.5 mV pH-1,
indicating that the electro-chemical reaction involves equal
numbers of proton-transfer and electron-transfer.
Fig. 4B shows the influence of preconcentration time on the
current response of quercetin at PtNPs/PEDOT-MeOH/GCE. The
PtNPs/PEDOT-MeOH/GCE was dipped into 50 mmol L-1 under
different preconcentration times. The anodic peak current
increased rapidly with the increasing of preconcentration time
and reached a platform at 80 s. The rapid response of quercetin at
PtNPs/PEDOT-MeOH/GCE was attributed to the synergic effect of
excellent conductivity of PEDOT-MeOH and the catalytic activity of
PtNPs. Thus,80 s of preconcentration time was employed in the
experiments.
3.4. Effect of scan rates toward quercetin
The effect of scan rates on the electrochemical behavior of
quercetin at PtNPs/PEDOT-MeOH/GCE was investigated by CV
(Fig. 5A). As shown in Fig. 5B,with the increasing of scan rates from
10 to 300 mV s-1,the reversible anodic peak currents (Ipa) and
cathodic peak currents (Ipc) increased linearly with the scan rates
(n). The regression equations were Ipa (mA) = 0.54n + 10.78 (n in
mV s-1) (R2 = 0.9951) and Ipc (mA) = 0.42n + 2.16 (R2 = 0.9989),
indicating that the reaction is an adsorption-controlled process.
3.5. Detection of quercetin
DPV method was selected for determination trace amounts
of quercetin in 0.1 mol L-1 PBS (pH 7.0). Fig. 6 shows DPV
responses of different quercetin concentrations at PEDOT-MeOH/
GCE and PtNPs/PEDOT-MeOH/GCE under the optimum experimental
conditions,which were preconcentrated in quercetin
solutions with different concentrations for 80 s. As can be seen
from Fig. 6A,the linear response ranges of quercetin at PEDOTMeOH/
GCE were 0.1-1.0 mmol L-1 and 1.0-31.0 mmol L-1. The
detection limit is found to be 0.015 mmol L-1 (S/N = 3). For PtNPs/
PEDOT-MeOH/GCE (Fig. 6B),the peak currents were proportional
to the concentration of quercetin in three ranges,0.04-
1.0 mmol L-1,1.0-19.6 mmol L-1 and 19.6-90.9 mmol L-1. The
linearization equations were I1 (mA) = 48.48 + 9.51C (mmol L-1)
(R2 = 0.9452),I2 (mA) = 56.86 + 1.10C (mmol L-1) (R2 = 0.9912)
and I3 (mA) = 71.18 + 0.31C (mmol L-1) (R2 = 0.9992),respectively,
with a detection limit of 5.2 nmol L-1 (S/N = 3). Compared with
PEDOT-MeOH/GCE,PtNPs/PEDOT-MeOH/GCE has a lower detection
limit,indicating that the introduction of PtNPs can greatly
improve the electrocatalytic activity toward quercetin. A comparison
of linear range,detection limit,and preconcentration time
at PtNPs/PEDOT-MeOH/GCE with other quercetin sensors
reported in the literature are shown in Table 2. The preconcentration
time at PtNPs/PEDOT-MeOH/GCE exhibited much lower
time than other previously reported electrodes [8, 9, 10, 12, 31],
revealing the rapid response of quercetin at PtNPs/PEDOT-MeOH/
GCE. Meanwhile,the detection limit was lower than some other
previously reported electrodes [8, 9]. In combination of the
electrocatalytic activity and strong adsorption ability of nanoparticles
PtNPs with the advantages of conducting polymer (good
water solubility and high conductivity),the higher catalytic
efficiency and stronger adsorptive ability of PtNPs/PEDOT-MeOH/
GCE exhibited excellent performances for the trace determination
of quercetin.
3.6. Reproducibility,stability and selectivity of PtNPs/PEDOT-MeOH/
GCE
The reproducibility of PtNPs/PEDOT-MeOH/GCE for the electrochemical
response of quercetin was estimated by 30th
successive measurements with the relative standard deviation
(RSD) of 0.67%. The RSD of five individual determination of MP was
3.5%,indicating an excellent reproducibility of PtNPs/PEDOTMeOH/
GCE.
The stability of PtNPs/PEDOT-MeOH/GCE was also explored.
After 10 days of storage,the response to quercetin was tested each
day with the response of the sensor decreasing only 5% compared
to the initial response,which confirms long-term stability.
Moreover,under the optimal experimental conditions,interference
studies were carried out by adding various foreign species
to a fixed amount of quercetin (50 mmol L-1). The results showed
that ions,such as K+,Ca2+,Na+,Mg2+,Pb2+,Ni2+,Zr2+,Fe3+,Cu2+,SO42-,PO43-,NO3-,and NO2- at a 100-fold concentration,glucose,
l-tyrosine,glycine,folic acid,and citric acid in a 50-fold
concentration,ascorbic acid at a 20-fold concentration had no
effect on the detection of quercetin. The change of the peak
currents of quercetin was less than 5% (i.e.,96.6%-104.3%),
suggesting that the proposed electrode has good selectivity.
4. Conclusion
A simple and sensitive quercetin electrochemical sensor was
successfully constructed based on conducting polymer PEDOTMeOH
and nanoparticles PtNPs. The synergic effect of the
electrocatalytic activity and strong adsorption ability of PtNPs
with the advantages of PEDOT-MeOH (good water solubility
and high conductivity) exhibited high catalytic efficiency and
strong adsorptive ability for the trace determination of quercetin.
The sensitivity of the electrochemical sensor was 9.51 mA
mol L-1 cm-2 and the limit of detection was 5.2 nmol L-1.
Furthermore,the modified electrode also exhibited good reproducibility
and long-term stability,as well as high selectivity.
Acknowledgments
This work was supported by the National Natural Science
Foundation of China (Nos. 51263010 and 51272096),Jiangxi
Provincial Department of Education (No. GJJ11590),Natural
Science Foundation of Jiangxi Province (No. 2010GZH0041) and
Graduate Student Innovation Foundation of Jiangxi Province (No.
YC2012-S123).
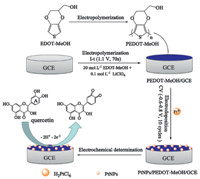
Scheme 1.The process of construct PtNPs/PEDOT-MeOH/GCE and trace determination of quercetin.
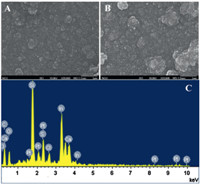
Fig. 1.SEM image of the PEDOT-MeOH film (A) and PtNPs/PEDOT-MeOH film (B),and EDX of PtNPs/PEDOT-MeOH film (C).
![]()
Table 1
Data of elemental analysis of PtNPs/PEDOT-MeOH from EDX.

Fig. 2.(A) Nyquist plots of different electrodes in 5 mmol L-1 [Fe(CN)6]3-/4- containing 0.1 mol L-1 KCl: bare GCE (a),PEDOT-MeOH/GCE (b),and PtNPs/PEDOT-MeOH/GCE(c). (B) CVs of different electrodes in 5 mmol L-1 [Fe(CN)6]3-/4- containing 0.1 mol L-1 KCl: bare GCE (a),PEDOT-MeOH/GCE (b),and PtNPs/PEDOT-MeOH/GCE (c). Scan rate:50 mV s-1.
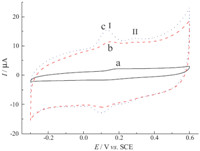
Fig. 3.CVs of 50 mmol L-1 quercetin in 0.1 mol L-1 PBS (pH 7.0) at different electrodes: bare GCE (a),PEDOT-MeOH/GCE (b),and PtNPs/PEDOT-MeOH/GCE (c).Scan rate: 50 mV s-1.

Scheme 2.The mechanism of oxidation of quercetin.

Fig. 4.Effect of (a) the pH value and (b) preconcentration time for the adsorption of quercetin at PtNPs/PEDOT-MeOH/GCE in 0.1 mol L-1 PBS (pH 7.0).

Fig. 5.(a) CVs of 50 mmol L-1 quercetin at PtNPs/PEDOT-MeOH/GCE with different scan rates: 10,20,30,50,70,100,150,200,250 and 300 mV s-1 (from a to j). (b) The plot of peak currents vs. scan rate.

Fig. 6.DPVs of PEDOT-MeOH/GCE in different concentrations of quercetin (0.1,0.4,0.8,1.0,3.0,5.5,9.0,13.8,19.0,and 31.0 mmol L-1) (A). DPVs of PtNPs/PEDOT-MeOH/GCE
in different concentrations of quercetin (a-o: 0,0.04,0.06,0.1,0.4,0.8,1.0,3.0,5.5,9.0,13.8,19.0,39.1,65.6,and 90.9 mmol L-1) (B). Inset: Plot of peak current vs.concentration of quercetin. Supporting electrolyte: 0.1 mol L-1 PBS (pH 7.0); accumulation time: 80 s; potential increment: 4 mV.
![]()
Table 2
Comparison of the proposed electrode with other reported electrodes for the determination of quercetin.
| [1] | N.C. Cook, S. Samman, Flavonoids - chemistry, metabolism, cardioprotective effects, and dietary sources, J. Nutr. Biochem. 7 (1996) 66-76. |
| [2] | A.K. Verma, J.A. Johnson, M.N. Gould, M.A. Tanner, Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin, Cancer Res. 48 (1988) 5754-5758. |
| [3] | P. Xiao, F.O. Zhao, B.Z. Zeng, Voltammetric determination of quercetin at a multiwalled carbon nanotubes paste electrode, Michrochim. J. 85 (2007) 244-249. |
| [4] | E. Ranjbari, P. Biparva, M.R. Hadjmohammadi, Utilization of inverted dispersive liquid-liquid microextraction followed by HPLC-UV as a sensitive and efficient method for the extraction and determination of quercetin in honey and biological samples, Talanta 89 (2012) 117-123. |
| [5] | D.G. Watson, E.J. Oliveira, Solid-phase extraction and gas chromatography-mass spectrometry determination of kaempferol and quercetin in human urine after consumption of Ginkgo biloba tablets, J. Chromatogr. B 723 (1999) 203-210. |
| [6] | K. Ishii, T. Furuta, Y. Kasuya, High-performance liquid chromatographic determination of quercetin in human plasma and urine utilizing solid-phase extraction and ultraviolet detection, J. Chromatogr. B 794 (2003) 49-56. |
| [7] | A. Molinelli, R. Weiss, B. Mizaikoff, Advanced solid phase extraction using molecularly imprinted polymers for the determination of quercetin in red wine, J. Agric. Food Chem. 50 (2002) 1804 1808. |
| [8] | X.Q. Lin, J.B. He, Z.G. Zha, Simultaneous determination of quercetin and rutin at a multi-wall carbon-nanotube paste electrodes by reversing differential pulse voltammetry, Sens. Actuators B 119 (2006) 608-614. |
| [9] | J.B. He, X.Q. Lin, J. Pan, Multi-wall carbon nanotube paste electrode for adsorptive stripping determination of quercetin: a comparison with graphite paste electrode via voltammetry and chronopotentiometry, Electroanalysis 17 (2005) 1681- 1686. |
| [10] | G.P. Jin, J.B. He, Z.B. Rui, F.S. Meng, Electrochemical behavior and adsorptive stripping voltammetric determination of quercetin at multi-wall carbon nanotubes- modified paraffin-impregnated graphite disk electrode, Electrochim. Acta 51 (2006) 4341-4346. |
| [11] | A.C. Oliveira, L.H. Mascaro, Evaluation of carbon nanotube paste electrode modified with copper microparticles and its application to determination of quercetin, Int. J. Electrochem. Sci. 6 (2011) 804-818. |
| [12] | M.S. Tehrani, A. Pourhabib, S.W. Husanin, M. Arvand, Electrochemical behavior and voltammetric determination of quercetin in foods by graphene nanosheets modified electrode, Anal. Bioanal. Electrochem. 5 (2013) 1-18. |
| [13] | S. Hrapovic, Y.L. Liu, K.B. Male, Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes, Anal. Chem. 76 (2004) 1083-1088. |
| [14] | S.J. Guo, D. Wen, Y.M. Zhai, S.J. Dong, E. Wang, Platinum nanoparticle ensembleon- graphene hybrid nanosheet: one-pot, rapid synthesis, and used as new electrode material for electrochemical sensing, ACS Nano 4 (2010) 3959-3968. |
| [15] | D.W. Hatchett, M. Josowicz, Composites of intrinsically conducting polymers as sensing nanomaterials, Chem. Rev. 108 (2008) 746-769. |
| [16] | Rajesh, T. Ahuja, D. Kumar, Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications, Sens. Actuators B 136 (2009) 275-286. |
| [17] | C. Li, H. Bai, G.Q. Shi, Conducting polymer nanomaterials: electrosynthesis and applications, Chem. Soc. Rev. 38 (2009) 2397-2409. |
| [18] | B.T. Li, L.M. Tang, K. Chen, et al., Coordinated organogel templated fabrication of silver/polypyrrole composite nanowires, Chin. Chem. Lett. 22 (2011) 123-126. |
| [19] | L.R. Wang, F. Ran, Y.T. Tan, et al., Coral reef-like polyanaline nanotubes prepared by a reactive template of manganese oxide for supercapacitor electrode, Chin. Chem. Lett. 22 (2011) 964-968. |
| [20] | E. Poverenov, M. Li, A. Bitler, M. Bendikov, Major effect of electropolymerization solvent on morphology and electrochromic properties of PEDOT films, Chem. Mater. 22 (2010) 4019-4025. |
| [21] | J. Jang, M. Chang, H. Yoon, Chemical sensors based on highly conductive poly(3,4- ethylenedioxythiophene) nanorods, Adv. Mater. 17 (2005) 1616-1620. |
| [22] | L. Groenendaal, G. Zotti, F. Jonas, et al., Electrochemistry of poly(3,4-ethylenedioxythiophene) derivatives, Adv. Mater. 15 (2003) 855-879. |
| [23] | A. Elschner, S. Kirchmeyer, W. Lövenich, U. Merker, K. Reuter, PEDOT: Principles and Applications of an Intrinsically Conductive Polymer, vol. 10, CRC Press/Taylor & Francis Group, Boca Raton/London/New York, 2011. |
| [24] | Y.P. Wen, L.M. Lu, D. Li, et al., Ascorbate oxidase electrochemical biosensor based on the biocompatible poly(3,4-ethylenedioxythiophene) matrices for agricultural application in crops, Chin. Chem. Lett. 23 (2012) 221-224. |
| [25] | A. Lima, P. Schottland, S. Sadki, C. Chevrot, Electropolymerization of 3,4-ethylenedioxythiophene and 3,4-ethylenedioxythiophene methanol in the presence of dodecylbenzenesulfonate, Synth. Met. 93 (1998) 33-41. |
| [26] | Y.H. Xiao, X.Y. Cui, J.M. Hancock, et al., Electrochemical polymerization of poly(hydroxymethylated-3,4-ethylenedioxythiophene) (PEDOT-MeOH) on multichannel neural probes, Sens. Actuators B 99 (2004) 437-443. |
| [27] | L.P. Wu, L.M. Lu, L. Zhang, et al., Electrochemical determination of the anticancer herbal drug shikonin at a nanostructured poly(hydroxymethylated-3,4-ethylenedioxythiophene) modified electrode, Electroanalysis 25 (2013) 2244-2250. |
| [28] | Y.P. Wen, D. Li, Y. Lu, et al., Poly(3,4-ethylenedioxythiophene methanol)/ascorbate oxidase/nafion-single-walled carbon nanotubes biosensor for voltammetric detection of vitamin C, Chin. J. Polym. Sci. 30 (2012) 824-836. |
| [29] | Y. Lu, Y.P. Wen, B.Y. Lu, et al., Electrosynthesis and characterization of poly(hydroxymethylated- 3,4-ethylenedioxythiophene) film in aqueous micellar solution and its biosensing application, Chin. J. Polym. Sci. 30 (2012) 824-836. |
| [30] | A.M.O. Brett, M.E. Ghica, Electrochemical oxidation of quercetin, Electroanalysis 15 (2003) 1745-1750. |
| [31] | G.R. Xu, S. Kim, Selective determination of quercetin using carbon nanotubemodified electrodes, Electroanalysis 18 (2006) 1786-1792. |






