Since the concept of host-guest chemistry was firstly proposed by Donald J. Cram [1],the research based on the host-guest chemistry has rapidly developed and attracted great interest [2, 3, 4, 5, 6, 7]. Cyclodextrin is a well-known class of host molecules and has advantages in its chiral cavity over crown and calyx[n]arene derivatives and therefore,cyclodextrin can be used to fabricate many chiral supramolecular systems [8]. Due to the specific shape of cyclodextrin hydrophobic cavities,cyclodextrin is capable of encapsulating small molecules. As reported,gamma-cyclodextrin (γ-CyD) with eight D-glucopyranose units,respectively,linked by a-(1,4) linkages can form the host-gust complex with pyrene, anthracene,phenanthrene [9, 10, 11] or steroidal compounds [12]. The host-guest interaction between cyclodextrin and small molecules had wide range of applications,such as promoting organic reactions [13, 14] and the stereoselectivity of photoreactions [15, 16, 17, 18],constructing stimuli-responsive supramolecular assemblies [19],fabricating functional materials [20] and mimicking the natural enzymatic systems [21]. On the other hand,the Langmuir-Blodgett (LB) technique is an important twodimensional method to fabricate supramolecular ultra-thin films and showed some merits to regulate the self-assembly of amphiphiles at the air/water interface [22, 23, 24, 25]. Some wellorganized chiral supramolecular assemblies from achiral molecules containing cyclodextrins have been constructed through the host-guest interaction on the interface [26]. However,there are still few reports about the two-dimension interfacial self-assembly of atypical amphiphiles without long alkyl chains and in situ photoreactions in the hydrophobic cavity of cyclodextrins [27, 28]. In this article,we designed a series of isomeric anthracenecontaining L-glutamate dendrons,which differ in the substituted position on the anthracene ring. With the appropriate balance between the focal and peripheral groups,the amphiphilic dendron, N-(2-anthracenecarboxyl)-1,5-bis(L-glutamic acid diethylester)-Lglutamic diamide (2-AGE),could form the stable monolayer with γ-CyD through the host-guest interaction and photodimerization of 2-AGE dendron occurred in situ inside the cavities of γ-CyD. In contrast,due to the differences of substituted sites,the host-guest complex was not obtained between N-(9-anthracenecarboxyl)- 1,5-bis(L-glutamic acid diethylester)-L-glutamic diamide (9-AGE) and γ-CyD. 2. Experimental
The synthesis of 2-AGE and 9-AGE was reported by us previously [29]. γ-CyD was purchased by Sigma-Aldrich. Deionized water used in our experiment was purified by Millipore Q system (18.2 MΩ cm). Chloroform used as spreading solvent is of analytical grade. The ultra-thin films of these dendritic compounds were fabricated by spreading 100 μL chloroform solution (ca. 0.326 mmol/L) on pure water,or aqueous solution containing 1 mmol/L γ-CyD for 1 h. The surface pressure-area (π-A) isotherms were measured with a KSV film balance with a compression speed of 12 cm2/min at 20.0 ± 0.2℃. In order to observe the surface morphology,the LB films were deposited onto a newly cleaved mica surface at different surface pressures (0 mN/m, 5 mN/m and 15 mN/m) and the AFM images were measured with a Digital Instrument Nanoscope III Multimode system (Santa,Barbara, CA) with a silicon cantilever using the tapping mode. All the AFM images are shown in the height mode without any image processing, except flattening. The temperature was keep at 20℃ when these films were transferred. The LS films were produced by transferring the monolayer onto quartz and CaF2 plates at a constant surface pressure of 5 mN/m through a horizontal lifting method. The UV/vis spectra were recorded with a JASCO UV-530 system. Circular dichroism (CD) spectra of the films were attained on a JASCO J-810 CD spectrometer. In the process of measurement,the films were placed perpendicular to the light path and rotated within the film plane to avoid polarization-dependent reflections and eliminate the possible angle dependence of the CD signals [30]. All the CD spectra are reproducible and no significant variations are observed for two identical samples. FTIR spectra were obtained on a Jasco FT/IR-660 plus spectrophotometer. The measurement was carried out over the scanning range from 1000 cm-1 to 4000 cm-1. Furthermore,in situ UV irradiation method used in our experiment was performed on the spreading films on γ-CyD surface by a 25W UV lamp (365 nm) for 1 h,which was hung over the film at a distance of 15 cm. 3. Results and discussion
Fig. 1 shows the π-A isotherms of the dendritic compounds spread on a different subphase and with different methods. For 2- AGE spread on aqueous solution containing γ-CyD,the isotherm showed an onset surface pressure at 1.2 nm2 per molecule. With the increasing of the surface pressure and although the L-glutamate dendron has no substituted alkyl chains,it can still form the stable Langmuir monolayer at the air/water interface. In comparison with the 2-AGE spreading on water which has been investigated previously [29],we found that an inflection point appearing at about surface pressure 8 mN/m on water was nonexistent when the subphase was changed to γ-CyD. These different isotherms indicated that there was an interaction between 2-AGE molecules and γ-CyD. However,after in situ irradiation of the process for 1 h, the isotherms showed a bad surface activity with the maximum surface pressure less than 20 mN/m. It was obviously know that in situ irradiation damaged the hydrophobic effect between the anthracene rings. With the increase of irradiation time,the hydrophobicity of the ultrathin films decreased. Another dendritic molecule 9-AGE also showed a slight surface activity at a very lower molecular area,indicating that 9-AGE did not form a stable monolayer on γ-CyD subphase.

|
Download:
|
| Fig. 1.Surface pressure-area (p-A) isotherms of the dendron compounds at 20 8C (a) 2-AGE,on pure water; (b) 2-AGE,on γ-CyD; (c) 9-AGE,on γ-CyD; (d) 2-AGE,on γ- CyD with in situ irradiation. The concentration of γ-CyD subphase is 1 mmol/L. The inset is the molecular structure of 2-AGE and 9-AGE. | |
To further disclose the differences in these systems,the ultrathin films of dendritic molecules were deposited on the mica surface by a vertical up-take with a speed of 2 mm/min and their morphology was detected. We have divided the isotherms into three pressure regions of film deposition. Region I is for the monolayer at a surface pressure of ca. 0 mN/m. Region II corresponds to the middle part of the isotherm where the surface pressure is between 3 mN/m and 5 mN/m. Region III represents the region above the second transition. Fig. 2A-C shows the AFM images of 9-AGE deposited from the γ-CyD subphase at different regions. The nanodots and nanosheets through the stacking of the nanodots were observed at Region I. With the increasing of surface pressure,the nanodots closely gathered to form the nanoparticles at Regions II and III,which suggested that there was no change on self-assembly of 9-AGE. When 2-AGE was spread on γ-CyD subphase,nanosheet structures with some nanodots were also formed at Region I. To our surprise,2-AGE assembled into curly nanofiber structure for the film deposited at Region II. The nanofibers have an average height of about 1.7 nm as well as extending to several micrometers,which is similar to the morphology of 2-AGE deposited on pure water [27]. However, some nanodots were also observed at this state. When it compressed the monolayer further to 15 mM/m,the nanofibers stacked densely and the nanoparticles decreased. This result indicated that γ-CyD actually had influence on the self-assembly of 2-AGE at Region I,but the effect was dominated by surface pressure. Interestingly,all the nanofiber structures obtained at higher surface pressure had disappeared,and only remaining nanoparticles after in situ irradiation. The size of the nanoparticles is about 0.2-0.5 μm and the average height is about 2 nm. With the increases of surface pressure,the nanoparticles arranged tightly and even overlapped.

|
Download:
|
| Fig. 2.AFM images of one-layer LB films of the dendron compounds transferred from 1 mmol/L γ-CyD aqueous solution subphase of (A-C) 9-AGE at 0,5,15 mN/m; (D-F) 2- AGE at 0,5,15 mN/m; (G-I) 2-AGE with in situ irradiation at 0,5,15 mN/m. | |
In order to confirm the interaction between 2-AGE and γ-CyD, FT-IR spectra of LS films were measured (Fig. 3A). In the FTIR spectra of 2-AGE LS films obtained on γ-CyD subphase,strong vibration bands are observed at 3298,1730,1638,and 1539 cm-1, which were assigned to N-H stretching vibration,ester carbonyl stretching,and amide I and II,respectively. Compared with FTIR spectra of 2-AGE LS films fabricated under in situ irradiation and γ- CyD,a broad peak at 3356 cm-1 was obviously observed due to the overlap of N-H and O-H stretching vibration,and further provided evidence that γ-CyD existed at the LS films. Moreover,the appearance of vibration band at 1474 cm-1 evidently confirmed the formation of anthracene dimers,as was reported for hydrocarbon cages [31, 32]. Additionally,the amide II band was broaden by the formation of dimers and amide I was shifted to higher wavenumbers,suggesting the weaken hydrogen bonds with in situ irradiation [33] in the LS films. In the UV/vis spectra of 2-AGE LS films (Fig. 3B),two absorption peaks of the anthracene group at 249 nm and 414 nm were observed,indicating that Haggregates of the chromophore were in the film state [29]. Moreover,the CD spectrum with a positive Cotton effect in the range from 290 nm to 200 nm was measured. The position of Cotton effect is consistent with the absorption maximum of the compound,indicating that a supramolecular chirality is obtained for the films. Such chirality was transferred from the chiral center of L-glutamic acid rather than from the chiral cavity of γ-CyD, which further suggests that the anthracene group was squeezed out of the γ-CyD cavity at a higher surface pressure. In comparison with 2-AGE LS films,the films obtained from in situ irradiation showed a significant decrease at 249 nm,suggesting that the in situ irradiation film underwent a extensive photodimerization reaction. Interestingly,no CD signal was observed after in situ irradiation,compared with the CD split of in situ irradiation 2- AGE LS films fabricated on the pure water subphase. Considering the FTIR spectrum,this result indicated that here the assembly of host-guest complex was entirely disordered and γ-CyD,as a silencer,blocked the supramolecular chirality transfer of 2-AGE assemblies.

|
Download:
|
| Fig. 3.(A) FT-IR spectra of 2-AGE LS films transferred from γ-CyD subphase at 5 mN/m (a) without; (b) with in situ UV irradiation; and (c) γ-CyD. (B) CD (top) and UV/vis (bottom) spectra of 2-AGE LS films transferred from γ-CyD subphase at 5 mN/m (a) without; (b) with in situ UV irradiation. | |
Based on the above characterization data,a possible mechanism was proposed as shown in Scheme 1. When 2-AGE was dispersed on the subphase containing γ-CyD,a host-guest reaction could occur at the air/water interface,as shown in Scheme 1A. During such a reaction,the two hydrophobic anthracene groups of 2-AGE were pulled into the cavity of γ-CyD. The host-guest reaction between the anthracene group and γ-CyD is related to the substituted position of anthracene group. Therefore,we obtained nanosheets of 2-AGE assemblies containing γ-CyD at lower surface pressure. In contrast,no great change was observed in 9-AGE assemblies at low and high surface pressure. Interestingly,upon compression,the anthracene group can be squeezed out of the γ- CyD cavity and assembled through π-π stacking. We observed the nanofibers,in the case of the 2-AGE monolayer on the γ-CyD subphase,at a higher surface pressure with this hypothesis further supported by the FTIR data. The anthracene group is photoactive, and can form a dimer after the irradiation. In the case of in situ irradiation method,similarly,two anthracene groups were wrapped by the cavity of γ-CyD. After the irradiation,the photodimerization of 2-AGE occurred at the cavity of γ-CyD and a host-guest complex containing γ-CyD was formed,as shown in Scheme 1B. In this case,the irregular nanoparticle structures were obtained through the disordered assembly. Moreover,these host-guest complexes are stable enough and their assembled nanostructures were not influenced by the change of surface pressure.
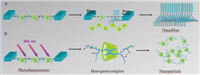
|
Download:
|
| Scheme 1.Schematic illustration of the interfacial assembly methods of 2-AGE. (A) on the surface of γ-CyD,2-AGE and γ-CyD form inclusion complexes at low surface pressure,but further compression will destroy the complexes and 2-AGE reassembled into nanofibers. (B) In situ irradiation stabilized the complexes and further irregularly assembled into nanoparticles. | |
Two kinds of atypical amphiphilic molecules based L-glutamate dendron were synthesized. Even without long alkyl chains,it has been proved that they could still form the stable Langmuir monolayer at the air/water interface. Because of the specific cavity of γ-cyclodextrin and the hydrophobic interaction between the anthracene rings,the 2-anthracenecarboxyl substituted dendron could form the host-guest complex with γ-cyclodextrin at the air/ water interface. This complex was unstable and it was easily affected by the change of the surface pressure. However,with the increase of surface pressure,2-AGE escaped from cavity of gcyclodextrin, and organized into nanofiber structures throughπ-π stacking. Dimerization was observed at the air/water interface by in situ irradiation method and stable dimer of 2-AGE encapsulated by γ-cyclodextrin was formed,which self-assembled into irregular nanoparticles. Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21021003 and 50673095),the Basic Research Development Program (Nos. 2007CB808005 and 2009CB930802),and the Fund of the Chinese Academy of Sciences.P
| [1] | D.J. Cram, J.M. Cram, Host-guest chemistry, Science 183 (1974) 803-809. |
| [2] | L.H. Tong, Cyclodextrin Chemistry: Basis and Application, Scientific Press, Beijing, 2001. |
| [3] | M.L. Bender, M. Komiyama, Cyclodextrin Chemistry, Springer-Verlag, Berlin; Heidelberg; New York, 1978. |
| [4] | S. Jozsef, Cyclodextrin Technology, Kluwer Academic Publishers, Dordrecht; Boston; London, 1988. |
| [5] | J. Szijfli, T. Osa, Comprehensive Supramolecular Chemistry: Cyclodextrin, Pergamon, Oxford, UK, 1996. |
| [6] | F. Hapiot, S. Tilloy, E. Monflier, Cyclodextrins as supramolecular hosts for organometallic complexes, Chem. Rev. 106 (2006) 767-781. |
| [7] | G. Wenz, B.H. Han, A. Muller, Cyclodextrin rotaxanes and polyrotaxanes, Chem. Rev. 106 (2006) 782-817. |
| [8] | Y.A. Zhdanov, Y.E. Alekseev, E.V. Kompantseva, Induced optical-activity in cyclodextrin complexes, Russ. Chem. Rev. 61 (1992) 563-575. |
| [9] | A. Ueno, I. Suzuki, T. Osa, Host-guest sensory systems for detecting organic compounds by pyrene excimer fluorescence, Anal. Chem. 62 (1990) 2461-2466. |
| [10] | A. Munoz de la Pena, T. Ndou, J.B. Zung, I.M. Warner, Stoichiometry and formation constants of pyrene inclusion complexes with beta- and gamma-cyclodextrin, J. Phys. Chem. 95 (1991) 3330-3334. |
| [11] | F. Vogtle, W.M. Muller, Complexes of gamma-cyclodextrin with crown ethers, cryptands, coronates and cryptates, Angew. Chem. Int. Ed. 18 (1979) 623-624. |
| [12] | W. Saenger, Cyclodextrin inclusion compounds in research and industry, Angew. Chem. Int. Ed. 19 (1980) 344-362. |
| [13] | F.J. Duan, J.C. Ding, H.J. Deng, et al., An approach to the Paal-Knorr pyrroles synthesis in the presence of b-cyclodextrin in aqueous media, Chin. Chem. Lett. 24 (2013) 706-793. |
| [14] | D.R. Patil, D.S. Dalal, Biomimetic approach for the synthesis of N,N0-diarylsubstituted formamidines catalyzed by b-cyclodextrin in water, Chin. Chem. Lett. 23 (2012) 1125-1128. |
| [15] | A. Nakamura, Y. Inoue, electrostatic manipulate of enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate with γ-cyclodextrin cavity through chemical modification, J. Am. Chem. Soc. 127 (2005) 5338-5339. |
| [16] | C. Yang, T. Mori, Y. Inoue, et al., Highly stereoselective photocyclodimerization of a-cyclodextrin-appended anthracene mediated by γ-cyclodextrin and cucurbit[ 8]uril: a dramatic steric effect operating outside the binding site, J. Am. Chem. Soc. 130 (2008) 8574-8575. |
| [17] | D.A. Lightner, J.K. Gawronski, K. Gawronska, Conformational enantiomerism in bilirubin. Selection by cyclodextrins, J. Am. Chem. Soc. 107 (1985) 2456-2461. |
| [18] | C. Yang, Recent progress in supramolecular chiral photochemistry, Chin. Chem. Lett. 24 (2013) 437-441. |
| [19] | Y.P. Wang, N. Ma, Z.Q. Wang, X. Zhang, Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with a-cyclodextrin, Angew. Chem. Int. Ed. 46 (2007) 2823-2826. |
| [20] | Y. Liu, Y.L. Zhao, H.Y. Zhang, Recognition-induced supramolecular porous nanosphere formation from cyclodextrin conjugated by cholic acid, Langmuir 22 (2006) 3434-3438. |
| [21] | R. Kataky, E. Morgan, Potential of enzyme mimics in biomimetic sensors: a modified-cyclodextrin as a dehydrogenase enzyme mimic, Biosens. Bioelectron. 18 (2003) 1407-1417. |
| [22] | K.B. Blodgett, Films built by depositing successive monomolecular layers on a solid surface, J. Am. Chem. Soc. 57 (1935) 1007-1022. |
| [23] | G.G. Roberts, Langmuir-Blodgett Films, Plenum, New York, 1990. |
| [24] | M.C. Petty, Langmuir-Blodgett Films: An Introduction, Cambridge University Press, Cambridge, 1996. |
| [25] | D. Mobius, Molecular cooperation in monolayer organizates, Acc. Chem. Res. 14 (1981) 63-68. |
| [26] | Y.G. Li, M.H. Liu, Induced chirality of supramolecular assemblies of some amphiphiles with b-cyclodextrin through the interaction at the air/water interface, J. Colloid Interface Sci. 306 (2007) 386-390. |
| [27] | P.F. Duan, M.H. Liu, Self-assembly of L-glutamate based aromatic dendrons through the air/water interface: morphology, photodimerization and supramolecular chirality, Phys. Chem. Chem. Phys. 12 (2010) 4383-4389. |
| [28] | P.F. Duan, L. Qin, M.H. Liu, Langmuir-Blodgett films and chiroptical switch of an azobenzene-containing dendron regulated by the in situ host-guest reaction at the air/water interface, Langmuir 27 (2011) 1326-1331. |
| [29] | P.F. Duan, M.H. Liu, Design and self-assembly of L-glutamate-based aromatic dendrons as ambidextrous gelators of water and organic solvents, Langmuir 25 (2009) 8706-8713. |
| [30] | C. Spitz, S. Dähne, A. Ouart, H.W. Abraham, Proof of chirality of J-aggregates spontaneously and enantioselectively generated from achiral dyes, J. Phys. Chem. B 104 (2000) 8664-8669. |
| [31] | H. Bouas-Laurent, A. Castellan, J.P. Desvergne, R. Lapouyade, Photodimerization of anthracenes in fluid solution: structural aspects, Chem. Soc. Rev. 29 (2000) 43-55. |
| [32] | H. Bouas-Laurent, A. Castellan, J.P. Desvergne, Photodimerization of anthracenes in fluid solutions: (part 2) mechanistic aspects of the photocycloaddition and of the photochemical and thermal cleavage, Chem. Soc. Rev. 30 (2001) 248-263. |
| [33] | Q.M. Ji, R. Iwaura, T. Shimizu, Regulation of silica nanotube diameters: sol-gel transcription using solvent-sensitive morphological change of peptidic lipid nanotubes as templates, Chem. Mater. 19 (2007) 1329-1334. |




