b College of Life Science, Sichuan University, Chengdu 610064, China
Gymnotheca chinensis Decne,as one of the endemic genera of seed plants in China,is a perennial herb of family Saururaceae. The whole plants of G. chinensis have long been used as traditional herbal medicine to treat contusions and strains [1]. Our previous investigations on the constituents of G. chinensis had led to the isolation of 15 new lignans belonged to three kinds of lignan skeletons,namely dibenzocyclooctene,eupomatilone and eupodienone [2]. The latter two rare types were only previously isolated from the Australian shrub Eupomatia bennettii and Eupomatia laurina [3, 4, 5, 6, 7],respectively. Further investigation on the chemical constituents of G. chinensis resulted in the isolation of two new eupodienone lignans (Fig. 1). Compound 1 was tested for cytotoxic activity in HCT15,HCT116,A549,MCF-7 and HepG2 cells.

|
Download:
|
| Fig. 1.The structures of compounds 1 and 2. | |
Dried and powdered whole plant of G. chinensis (1.45 kg) was extracted with MeOH at r.t. to give an extract (152 g),which was suspended in H2O (1 L) and extracted with petroleum ether and AcOEt (3 × 1 L,3 h each) successively. The petroleum ether extract (8 g) and AcOEt extract (19 g) were combined and then subjected to CC over MCI gel (85 mm × 100 mm,MeOH/H2O 90:10). In total, two fractions (A and B) were obtained based on TLC analysis. Fraction A (15 g) was further separated by medium pressure CC (sio2 (49 mm × 460 mm),petroleum ether/acetone 100:1→1:1) to give twelve fractions. Fraction 11 was subjected to reversedphase CC with MeOH-H2O (70→100%) as the solvent system to yield four subfractions (11A–D). Compound 1 (25mg,tR = 19.0 min) was obtained from fraction 11A by semi-preparative HPLC (MeOH/H2O 60:40,flow rate 3.2mL/min). Fraction 9 was subjected to reversed-phase CC with MeOH-H2O (70→100%) as the solvent system to yield four subfractions (9A–D). Compound 2 (4 mg, tR = 21.7min) was obtained from fraction 9A by semi-preparative HPLC (CH3CN/H2O 50:50,flow rate 4.8mL/min). 3. Results and discussion
Compound 1 was obtained as a yellow gum,[α]D20 D t 20 (c 0.35, MeOH). Its molecular formula of C23H28O7,which indicated 10 degrees of unsaturation,was established from the quasi-molecular ion peak at m/z 439.1730 [M + Na]+ (calcd. for C23H28O7Na+: 439.1727) in the HRESIMS. Its IR spectrum exhibited strong absorption bands at 3436 and 1670 cm-1 due to hydroxyl and carbonyl functional groups. The 1H NMR and 13C NMR spectra (Table 1) showed that it probably belonged to the eupodienone family [2, 5]. The 1H NMR,13C NMR and HSQC spectra of 1 showed 23 carbon resonances due to two methyls [δH 1.03 (d,3H, J = 7.4 Hz),1.07 (d,3H,J = 7.2 Hz); δC 15.5,15.1],two oxygenated methines [δH 4.74 (s,1H),3.43 (d,1H,J = 2.7 Hz); δC 92.3,85.7] and other two methines [δH 2.33 (m,1H),2.77 (m,1H); δC 45.7,36.7], five methoxy groups [δH 3.57,3.71,3.84,3.56,3.69 (s,each 3H); δC 60.9,60.5,56.3,55.1,55.4],a quaternary carbon (δC 46.4),an aromatic ring moiety [δH 6.70 (s,1H); δC 154.1,153.8,142.4,135.7, 120.1,105.4],a diketene moiety [δH 6.20 (d,1H,J = 2.3 Hz),6.10 (d, 1H,J = 2.3 Hz); δC 118.8,150.3,175.6,153.9,121.9]. The HMBC (Fig. 2) correlations ofH-6/C-9 anδH-9/C-6 confirmed an epoxide at C-6 and C-9; key HMBC and HSQC correlations revealed that five methoxy groups were attached to the C-2,C-3,C-4,C-13 and C-15, respectively. The NOESY correlations of H-6/H3-17 and H-9/H3-18 supported the hypothesis that H-6,H-9,H3-17 and H3-18 existed in the same face of the molecule [2]. Detailed analysis of 1H NMR,13CNMR and HMBC spectra suggested that the structure of 1 was very similar to those of gymnothelignan O [2] except for the replacement of the methylenedioxy by two methoxy groups at C-2 and C-3. In summary,the structure of 1 was determined to be 6,9-epoxy- 7,8-dimethyl-2,3,4,13,15- pentamethoxy-10,11-benzospiro [5.6] dodec-13,15-dien-14-one and named as gymnothelignan T.
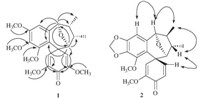
|
Download:
|
| Fig. 2.Key HMBC correlations of compound 1 and key NOESY correlations of compound 2. | |
Compound 2 was obtained as a white powder,[α]D20 D +17 (c 0.30, MeOH). The molecular formula was determined to be C21H22O6 by analysis of the HRESIMS ion peak atm/z 393.1300 [M + Na]+ (calcd. for C21H22O6Na+: 393.1309),which indicated 11 degrees of unsaturation. The 1H NMR and 13C NMR spectra (Table 1) were similar to those of 1. The HMBC correlations showed the presence of a methylenedioxy group at C-2 and C-3. HMBC correlations of H- 6/C-9 and H-9/C-6 displayed the connection of C-6 to C-9 via an ether bond. The vicinal coupling constants (H-9/H-8) value was 4.9 Hz for 2,whereas that of 1 was 0 Hz,indicating an epimerization at C-8. In a NOESY experiment (Fig. 2),the crosspeaks of H-7/H-16 indicated that H-7 and H-16 were cis to each other. Detailed HMBC analysis suggested that the structure of 2 was very similar to those of gymnothelignan N [2] except for the absence of the OCH3 at C-15,which was revealed by the 1H NMR ABX spin system (δH 7.08 (dd,1H,J = 10.1,2.4 Hz,H-16),6.39 (d, 1H,J = 10.1 Hz,H-15),6.13 (d,1H,J = 2.4 Hz,H-12). Compound 2 was then assigned as 6,9-epoxy-7,8-dimethyl-2,3-methylenedioxy- 4,13- dimethoxy-10,11-benzospiro [5.6] dodec-13,15- dien-14-one and named as gymnothelignan U.
| Table 1 1H NMR (400MHz) and13C NMR (100 MHz) data of compounds 1 and 2 (acetoned6). |
Two new eupodienone lignans,named gymnothelignan T (1) and gymnothelignan U (2) were isolated from the whole plant of G. chinensis. Compound 1 was tested for cytotoxic activity in HCT15, HCT116,A549,MCF-7 and HepG2 cells and exhibited no appreciable activity against these tested cell lines with IC50 values above 50 mmol/L. Acknowledgment
This work was financially supported by the grant from the National Natural Sciences Foundation of China (No. 30973634).
| [1] | Y.Q. Cheng, Flora of China, Science Press, Beijing, 1982, p. 9. |
| [2] | D.H. He, L.S. Ding, H.X. Xu, et al., Gymnothelignans A-O: conformation and absolute configuration analyses of lignans bearing tetrahydrofuran from Gymnotheca chinensis, J. Org. Chem. 77 (2012) 8435-8443. |
| [3] | B.F. Bowden, R.W. Read, W.C. Taylor, Constituents of Eupomatia species. VI. The structures of eupodienone-1,-2,-3, Aust. J. Chem. 33 (1980) 1823-1831. |
| [4] | B.F. Bowden, R.W. Read, W.C. Taylor, Constituents of Eupomatia species. Ⅶ. Dienone-phenol and dienol-benzene rearrangements in the eupodienone-1 series, Aust. J. Chem. 34 (1981) 799-817. |
| [5] | R.W. Read, W.C. Taylor, Constituents of Eupomatia species. Ⅷ. The structures of eupodienone-4,-5,-6,-7, Aust. J. Chem. 34 (1981) 1125-1134. |
| [6] | A.R. Carroll, W.C. Taylor, Constituents of Eupomatia species. XⅢ. The structures of new lignans from the tubexs and aerial parts of Eupomatia bennettii, Aust. J. Chem. 44 (1991) 1627-1633. |
| [7] | A.R. Carroll, W.C. Taylor, Constituents of Eupomatia species. XIV. The structures of eupomatilone-1,-2,-3,-4,-5,-6 and-7 isolated from Eupomatia bennettii, Aust. J. Chem. 44 (1991) 1705-1714. |





