1,2-Diols are important chemical raw materials for a wide variety of chemicals such as polyesters,surfactants,pharmaceutical raw materials and so on. The preparation of straight-chain 1,2- diols is mostly carried out by the epoxidation of olefins followed by the hydrolysis of the epoxides. The oxidation of olefins is the most significant step in preparing 1,2-diols,so it has attracted more and more attention in recent years [1, 2].
In the past decades,an extensively studied epoxidation system relevant to heteropolytungstophosphates was the Venturllo–Ishii system,but drawbacks of the Venturello–Ishii epoxidation are the use of chlorocarbons solvents and catalyst deactivation,causing difficulties in a catalyst cycle [3]. In order to solve this problem, heterogenization of such catalysts has been studied in many ways [4, 5, 6, 7, 8, 9, 10, 11, 12]. Recently,Xi reported a reaction-controlled phase-transfer catalysis for the epoxidation of olefins with H2O2 generated in situ or commercially available 30% H2O2 as the oxidant [13],Chen reported the nature of reaction-controlled phase transfer catalysts for the epoxidation of olefins using 31P NMR technique [14]. Hua reported [π-C5H5N(CH2)15CH3]3 [PMoW3O24],an efficient and recyclable heteropolyoxomolybdotungstate catalyst for the epoxidation of 1-octene with 30% H2O2 using environmentally friendly solvent [15]. However,the oxidation of olefins using H2O2 in the presence of carboxylic acids was scarcely studied,because peracids that was produced by the reaction of carboxylic acids and hydrogen peroxide (HP) are difficult to handle,but they are vital in the process. Formic acid (FA) can ionize under reaction conditions and the formation of performic acid (PFA) is fast enough even without the presence of mineral acids,a phenomena known as formic acid-autocatalyzed [16] preparation of PFA. PFA was generated by the reaction between FA and HP in aqueous phase and then quickly consumed in the epoxidation reaction in organic phase.
In thispaper,wereportour initial resultsonthe researchinwhich a tungsten-based polyoxometalate,[(C18H37)2N(CH3)2]3 [PW4O16] (abbreviated as Cat. I) was synthesized and used as a phase-transfer catalyst for the oxidation of terminal alkenes with H2O2 in the presence of carboxylic acids under solvent-free conditions. This systemcan be used for the catalytic oxidation of olefins and exhibits high conversion and yield. After reaction,the catalyst can be precipitated from the reaction medium and reused as a heterogeneous catalyst. Therefore,this catalytic system possesses the advantages of both heterogeneous and homogeneous catalysts. 2. Experimental
All solvents and chemicals were of analytical grade,commercially available and used without further purifications unless mentioned otherwise. 2.1. Preparation of catalysts
The catalyst [(C18H37)2N(CH3)2]3[PW4O16] (Cat. I) was prepared according to the literature [17]. A suspension of tungstic acid (5.0 g, 20 mmol) in 16.5 mL of 30% aqueous H2O2 was heated at 60°C for 15 min. H3PO4 (40%,1.24 mL,5.0 mmol) was added to the obtained colorless solution at room temperature and the whole solution was diluted with another 40 mL of water and then stirred for 30 min. To the resulted solution,6.0 g of dioctadecyl dimethyl ammonium chloride (10 mmol) in dichloromethane (80 mL) was added dropwise with stirring. After the solution was stirred continuously for 30 min,the organic phase was separated,dried over anhydrous Na2SO4,filtered and then the solvent was removed at 60 °C under atmospheric pressure. A yellow dry powder was obtained by further evaporating under reduced pressure.
The catalysts with different quaternary ammonium cations were similarly prepared using cetylpyridinium chloride (p- C5H5NC16H33Cl),hexadecyl trimethyl ammonium chloride (C16H33N(CH3)3Cl) and tetrabutylammonium hydrogen sulfate (Bu4NHSO4),denoted as Cat. II,Cat. III and Cat. IV,respectively. 2.2. Characterization techniques
The laser Raman experiments were performed using a Jobin Yvon ARAMIS,Raman spectrometer equipped with a CCD (Te RAMAN-1024 × 256-OPEN-SYN) detector and the spectrum resolution of 1 cm-1 using a laser at 532 nm as the excitation source. The infrared spectra were recorded on a Nicolet IS10 FT-IR spectrometer. 2.3. Catalytic oxidation of terminal alkenes to 1,2-diol
The catalytic reactions were performed in a 50 mL four-necked round-bottomed flask equipped with a reflux condenser. The assembly was kept in an isothermal water bath at a constant known temperature and mechanically agitated with an electric motor. The oxidation was carried out as follows: catalyst (0.12 mmol),terminal alkenes (30 mmol),H2O2 (30% aq.,40 mmol) and FA (60 mmol) were charged in the reaction flask. The catalyst/ olefin ratio was 1:250 for all the reactions. When the oxidation reaction was over,the catalyst was precipitated from the reaction medium for further use when acetone was subsequently added to the solution,the precipitate was removed by centrifugation and filtration,and the filtrate extracted with CH3Cl2 (50 mL × 3); the organic layer was collected and dried under anhydrous Na2SO4. After the evaporation of the solvent under reduced pressure,a solution of sodium hydroxide was added dropwise to the organic product to give high purity 1,2-diols. The organic product was analyzed by GC9600 using an internal standard method. Typical GC-MS analysis was also conducted to identify the products. 3. Results and discussion 3.1. Catalysts with different cations
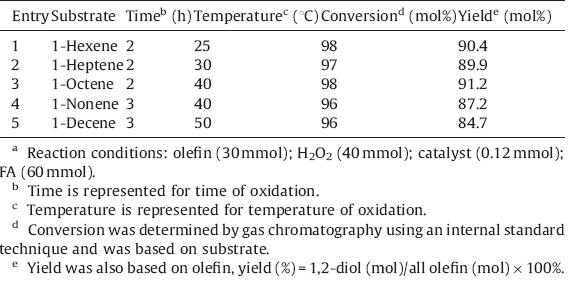
The oxidation of terminal olefins is the more difficult but important step in the alkene/diol transformation. In our catalytic system,the oxidation of terminal olefins generates some byproducts that can be transformed to the corresponding 1,2-diol in high yield (Scheme 1).
|
Download:
|
| Scheme 1.The process of phase-transfer catalysis for preparation of 1,2-diol. | |
Chen et al. [14] have reported that cations played a significant role in phase-transfer catalytic oxidation. It was found that the choice of proper cations is crucial for the precipitation of heteropoly species from the reaction media. Three more catalysts with different cations,[π-C5H5NC16H33]+,[C16H33N(CH3)3]+ and (Bu4N)+,were synthesized.
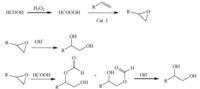
For catalyst Cat. I,its IR spectrum (Fig. 1A) gives a strong peak at 840 cm-1,which indicates that the catalyst still contain active oxygen. For catalyst Cat. II,its IR spectrum (Fig. 1D) indicates it is a mixture of [PW11O39]7- and [PW12O40]3-,as demonstrated by the following resonances: ν(PO4) 1077 cm-1; ν(W=O) 967 cm-1;n(W– Ob–W) 880 cm-1. The IR spectra of fresh catalysts showed almost no active oxygen (Fig. 1B and C). So the nature of quaternary ammonium cation affects the composition of the catalyst,and bigger cation can more efficiently stabilize active oxygen.
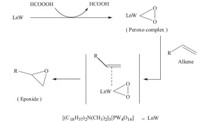
The oxidation of 1-octene catalyzed by a series of catalysts was conducted in the H2O2/formic acid system,and the results are summarized in Table 1. Cat. I showed the highest activity (entry 1) and a 91.2% yield of 1,2-octanediol was obtained. The activity of Cat. II was similar to that of Cat. I and a 80.4% yield of 1,2- octanediol was obtained. The activity of Cat. III and Cat. IV was not as good as that of Cat. I and the yields of 1,2-octanediol were 73.1% nd 60.6%,respectively (entries 3 and 4). The yield of 1,2- octanediol was only 51.9% in the absence of a catalyst (entry 5). The results indicate that the Cat. I is the optimal catalytic system. According to Ishii’s observations [18],a possible mechanism is given in Scheme 2 in the oxidation reaction of terminal alkenes.
|
Download:
|
| Fig. 1.IR spectra of the catalysts with different cations. (A) [(C18H37)2N(CH3)2]+; (B) [Bu4N]+; (C) [C16H33N(CH3)3]+; (D) [π-C5H5NC16H33]+. | |
| Table 1 Oxidation of 1-octene catalyzed by various catalyst.a |
|
Download:
|
| Scheme 2.The possible process of phase-transfer catalysis for preparation of epoxide. | |
After the catalytic oxidation of 1-octene in the medium of Cat.I/ H2O2/FA system,Cat. I could be precipitated from the reaction medium when acetone was subsequently added to the solution. Cat. I was recovered in 60% yield (by weight). One can observe from Table 2 that after four consecutive cycles of the reaction,a 78.6% yield of 1,2-diol with 74% conversion was still achieved using this catalytic system,but the yield of 1,2-diol decrease gradually from cycle to cycle. The decrease of the catalyst activity should be attributed to the loss of active oxygen.
| Table 2 Oxidation of 1-octene on the catalyst I for four consecutive cycles.a |
Fig. 2B presents the FT-IR spectrum of the used catalyst. It is obvious that there are differences in IR spectra between the fresh Cat. I and the used one. IR spectra show that the fresh Cat. I has one strong peroxo-bond absorption peak at v(O–O) at 846 cm-1,but the peroxo-bond disappeared for the recovered catalyst (Fig. 2B). Compared with the IR spectrum of the fresh Cat. I,the bands of used catalyst changed and are fairly similar to those of Keggin-type structure of the [PW11O39]7- anion and [PWl2O4]3- [19, 20]. Therefore,we concluded that new mixed-addenda polyanions with Keggin structure was produced after reaction.

|
Download:
|
| Fig. 2.IR spectra of (A) the fresh catalyst of Cat. I; (B) the used Cat. I. | |
Laser Raman spectra of the fresh and used catalysts in the solid state are shown in Fig. 3. According to the literature [21],the band at 1044 cm-1 is attributed to the quaternary ammonium cation. The band at 870 cm-1 is assigned to ν(O–O) (connected with W) [22]. The band at 870 cm-1 demonstrates that there is active oxygen in the structure of the fresh catalyst. The band at 560 cm-1 can be assigned to n(W(O2)).
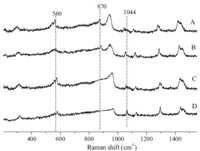
|
Download:
|
| Fig. 3.Raman spectra of (A) [(C18H37)2N(CH3)2]3[PW4O16]; (B) the catalyst of cycle 1; (C) the catalyst of cycle 2 and (D) the catalyst of cycle 3. | |
According to Fig. 3,for B,the Raman spectrum is very similar to that of fresh one,indicating most of the active functional group remained for the recovered catalyst. The band at 870 cm-1 disappeared (Fig. 3C and 3D). The results revealed that the structure of the catalyst-I has changed after catalytic reaction. 3.3. Catalytic oxidation of linear terminal olefins
[(C18H37)2N(CH3)2]3[PW4O16] (Cat. I) was used to catalyze the oxidation of a variety of terminal olefins using 30% H2O2 in the presence of FA. This oxidation system,Cat.I/H2O2/FA gives high catalytic activity and conversion (Table 3).
| Table 3 Oxidation of various linear terminal olefinsa |
In summary,we developed a phase-transfer catalytic system consisting of [(C18H37)2N(CH3)2]3[PW4O16]/HP/FA that can be used under solvent-free conditions. This system can efficiently catalyze terminal alkene oxidation in high yields in an environmentally friendly manner. The catalyst could be easily recovered and reused after reaction. This catalytic system overcomes the drawbacks of previously reported tungsten-based catalysts,which were difficulty to recover and required chlorocarbons solvents. After the reaction,part of species with high P/W ratios in the fresh catalyst changed to species with low P/W ratios. The quaternary ammonium cation used in the preparation process has a great impact on the composition of heteropoly tungstophosphates.
| [1] | B.S. Lane, K. Burgess, Metal-catalyzed epoxidations of alkenes with hydrogen peroxide, Chem. Rev. 7 (2003) 2457-2473. |
| [2] | G. Grigoropoutou, J.H. Clark, J.A. Elings, Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant, Green Chem. 5 (2003) 1-7. |
| [3] | D.C. Duncan, R.C. Chambers, E. Hecht, C.L. Hill, Mechanism and dynamics in the H3[PW12O40]-catalyzed selective epoxidation of terminal olefins by H2O2: formation, reactivity, and stability of {PO4][WO(O2)2]4}3, J. Am. Chem. Soc. 117 (1995) 681-691. |
| [4] | D. Yong, B.C. Ma, D.J. Tong, et al., Synthesis of epoxides catalyzed by a halide-free reaction-controlled phase-transfer catalytic system: [(CH3(CH2)17)2N(CH3)2]3[PW4O32]/H2O2/dioxan/olefin, Aust. J. Chem. 62 (2009) 739-746. |
| [5] | R. Neumann, H. Miller, Alkene oxidation in water using hydrophobic silica particles derivatized with polyoxometalates as catalysts, J. Chem. Soc. Chem. Commun. (1995) 2277-2278. |
| [6] | R. Neumann, M. Cohen, Solvent-anchored supported liquid phase catalysis: polyoxo-metalate-catalyzed oxidations, Angew. Chem. Int. Engl. 36 (1997) 1738-1740. |
| [7] | T. Sakamoto, C. Pac, Selective epoxidation of olefins by hydrogen peroxide in water using a polyoxometalate catalyst supported on chemically modified hydrophobic mesoporous silica gel, Tetrahedron Lett. 41 (2000) 10009-10012. |
| [8] | D. Yong, B.C. Ma, Q. Gao, J.S. Sou, Epoxidation of alkenes by hydrogen peroxide over 12-heteropolyacids of molybdenum and tungsten (H3PMo3W9O40) combined with cetylpyridinium bromide, J. Chem. Res. 2006 (2006) 499-503. |
| [9] | L. Hua, Y.X. Qiao, H. Li, et al., Epoxidation of olefins with hydrogen peroxide catalyzed by a reusable lacunary-type phosphotungstate catalyst, Sci. China Chem. 54 (2011) 769-773. |
| [10] | J. Chen, X. Chang, J.C. Jiang, et al., The research progress of the heteropoly acid quaternary ammonium salt phase transfer catalyst system, Guangzhou Chem. Ind. 40 (2012) 6-8. |
| [11] | J. Li, S. Gao, Z.W. Xi, Progress in reaction-controlled phase-transfer catalysis, Chin. J. Catal. 26 (2010) 895-911. |
| [12] | W.J. Xu, L. Jing, F.M. Zhang, et al., Research progress in phase-transfer catalysis by organic heteropolyacid salts, J. Zhejiang Normal Univ. 35 (2012) 85-92. |
| [13] | Z.W. Xi, N. Zhou, Y. Sun, K. Li, Reaction-controlled phase-transfer catalysis for propylene epoxidation to propylene oxide, Science 292 (2001) 1139-1141. |
| [14] | Y.Y. Chen, J.Q. Zhuang, X.M. Liu, et al., On the nature of reaction-controlled phase transfer catalysts for epoxidation of olefin: a 31P NMR investigation, Catal. Lett. 93 (2004) 41-46. |
| [15] | H. Hua, B.C. Ma, D.J. Tong, et al., [π-C5H5N(CH2)15CH3]3[PMoW3O24]: a heteropolyoxomolybdotungstate catalyst for efficient and recyclable epoxidation of 1-octene with 30% H2O2 using environmentally friendly solvent, J. Mol. Catal. A 23 (2009) 97-105. |
| [16] | X.Y. Sun, X.B. Zhao, W. Du, D.H. Liu, Kinetics of formic acid-autocatalyzed preparation of performic acid in aqueous phase, Chin. J. Chem. Eng. 19 (2011) 964-971. |
| [17] | G.D. Sala, A. Lattanzi, T. Severino, A. Scettri, The first application of titanocenes in the asymmetric oxidation of sulfides, J. Mol. Catal. A. 170 (2001) 219-224. |
| [18] | G.D. Yadav, A.A. Pujari, Epoxidation of styrene to styrene oxide: synergism of heteropoly acid and phase-transfer catalyst under Ishii-Venturello mechanism, Org. Proc. Res. Dev. 4 (2000) 88-93. |
| [19] | J. Gao, Y. Chen, Z. Xi, et al., A spectroscopic study on the reaction-controlled phase transfer catalyst in the epoxidation of cyclohexene, J. Mol. Catal. A: Chem. 210 (2004) 197-204. |
| [20] | C. Rocchiccioli-Deltchef, R. Thouvent, Metal complexes of heteropolyanions α-XM11O39n- with X = Si(IV) or P(V) and M = Mo(VI) or W(VI): study of structural modifications of ligand by infrared and Raman spectrometry, J. Chem. Res. (S) (1977) 46-47. |
| [21] | H. Chen, W.L. Dai, X.L. Yang, et al., Studies on the structural change of a reactioncontrolled phase-transfer [π-C5H5NC16H33]3{PO4][WO3]4} catalyst during the selective oxidation of cyclopentene to glutaric acid with aqueous H2O2, Appl. Catal. A: Gen. 309 (2006) 62-69. |
| [22] | L. Salles, C. Aubry, J.M. Bregeauh, et al., Preparation of various onium tetrakis (oxodiperoxomolybdo) phosphates. Ⅲ. Structure and reactivity towards olefins under biphasic conditions, New J. Chem. 17 (1993) 367-375. |