b Department of Chemistry, Deogiri College, Aurangabad 431 005, MS, India;
c Department of Chemistry, Dnyanopasak College, Parbhani 431 401, MS, India;
d Department of Chemistry, Sharda Mahavidyalaya, Parbhani 431 401, MS, India
Base-catalyzed condensation and addition reactions such as the Aldol,Knoevenagel and Michael reactions are well reported in the literature for the production of drugs,fragrances,and chemical intermediates [1, 2, 3, 4]. The use of novel environmentally friendly solid catalysts for the synthesis of biologically potent heterocyclic compounds has become one of the most active areas of research. Heterogeneous catalysts,particularly the solid-base catalysts synthesized by the functionalization of several solid-state materials are capable of catalyzing various C–C coupling reactions [5, 6]. Recently,mesoporous silica (OMS) and related materials have become popular as catalysts and adsorbents and have found extensive applications as containers for cluster/nanowire growth, etc. [7]. These materials have several advantages such as tunable pore diameter and high surface area,for which they have emerged as effective heterogeneous catalysts in organic synthesis [8].
Aminopropyl functionalized mesoporous silica materials are reported to be effective base catalysts for Knoevenagel condensation [9],Aldol condensation [10],Michael addition [9],epoxidation reactions [11],and Claisen–Schmidt condensations [12]. Furthermore, the development of biomimetic catalysts is also well reported in the literature [13]. The use of such heterogeneous catalysts offers several advantages in process development, particularly the separation of products from catalyst,and recycling of catalyst in reactor. Solid acidic and basic catalysts are alternatives for clean technologies and sustainable chemistry for different processes.
Tetrahydrochromene moiety is an important class of benzopyran derivatives found in many natural products [14]. More specifically,2-amino-4H-chromene-3-carbonitrile derivatives possess many biological activities such as anticancer,anticoagulant, antibacterial,and fungicidal properties [15]. These compounds find extensive applications in different fields,such as cosmetics,pigments,and agrochemicals [16]. Polyfunctionalized 4H-pyrans constitute the essential part of several natural products [17] and are also known as potential calcium channel antagonists [18]. As a result of widespread applications,the synthesis of these compounds has attracted a lot of interest. From the literature survey,numerous methods have been reported for the synthesis of tetrahydrochromene derivatives by one pot three component condensation of aldehyde,malononitrile and 1,3- dicarbonyl compounds using acidic or basic catalysts. Mola et al. documented borax catalyzed three component coupling of tetrahydrochromene in refluxing ethanol [19]. Silica-bonded N-propylpiperazine sodium n-propionate was employed as a recyclable catalyst for the synthesis of tetrahydrochromene derivatives [20]. Nano-ZnO catalyzed synthesis of dihydropyrano[ 2,3-c]chromenes can be accomplished in aqueousmedium[21]. The other methods used for the synthesis of these compounds involve basic catalysts such as piperidine [22],morpholine,pyridine [23],triethylamine [24] and sodium bromide [25]. The reported methods still have drawbacks such as long reaction time,high reaction temperature and use of toxic organic solvents. Therefore, development of a simpler synthesis method for chromene derivatives becomes essential. In the present studywe report the synthesis of 2-amino-5-oxo-4-phenyl-5,6-dihydro-4H-benzo[H]chromene- 3-carbonitrile derivatives by the reaction of aldehyde (1a) with 5,5-dimethylcyclohexane-1,3-dione (2) and malononitrile (3) at 70°C using aminopropylated SiO2 (AP-SiO2) as a polyamine heterogeneous base catalyst (Scheme 1).

|
Download:
|
| Scheme 1.Synthesis of 2-amino-5-oxo-4-phenyl-5,6-dihydro-4H-benzo[H]chromene-3-carbonitrile derivatives. | |
All solvents were used as commercial grade without further purification. Silica gel coated aluminium sheets (Merck made) were used for thin layer chromatography to monitor progress of reactions. The column chromatography was done over silica gel (80–120 mesh). Melting points were determined in an open capillary tube and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker 300 MHz spectrometer in CDCl3 solvent. Mass spectra were taken on Polaris-Q Thermoscientific GC–MS. Silica gel supported polyamine catalyst was prepared by a known literature process [27]. 2.1. General procedure for the synthesis of 4H-chromene derivatives
The mixture of aromatic aldehyde (1 mmol),5,5-dimethylcyclohexane- 1,3-dione 2 (1 mmol),and malononitrile 3 (1 mmol) was taken in water. To this mixture 10 mol% of solid aminopropylated SiO2 catalyst was added. The reaction mixture was stirred at 70 8C for the appropriate time (as shown in Table 1) and the progress of reaction was monitored by thin layer chromatography. After completion of reaction as indicated by TLC,the reaction mixture was cooled at room temperature. The solvent was removed under vacuum to obtain the crude product. To the crude product,ethyl acetate was added and the solid catalyst was filtered off. The organic layer was evaporated to obtain the solid product. The obtained product was purified by recrystallization using ethyl acetate to get the desired compound 4a–n. All compounds were characterized by comparing their spectral data with those reported in literature [15, 26, 27]. Spectral data for representative and newly synthesized compounds is listed below.
| Table 1 Synthesis of 4H-benzo[b]pyran derivatives using aminopropylated silica gel catalyst. |
2-Amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro- 4H-chromene-3-carbonitrile (4a): White solid,mp 235–237°C,1H NMR (300 MHz,CDCl3): δ 7.79–7.89 (m,2H),7.41–7.50 (m,1H), 6.88–7.04 (m,2H),6.57 (brs,2H,NH),4.29 (s,1H),2.58 (s,2H),2.26 (m,1H),2.01 (m,1H),1.23 (s,3H),0.84 (s,3H); 13C NMR (75 MHz, CDCl3): δ 201.2,162.3,155.8,145.6,128.7,127.5,119.2,112.1,57.6, 49.9,40.3,39.9,36.1,27.4; GC–MS: m/z 294 (M+); Elem. Anal. Calcd. for C18H18N2O2: C,73.45; H,6.16; N,9.52; O,10.87; found: C, 73.43; H,6.18; N,9.54; O,10.90.
2-Amino-7,7-dimethyl-5-oxo-4-(3,4,5-trimethoxyphenyl)- 5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4f): Yellow solid,mp 247–249°C,1H NMR (300 MHz,CDCl3): δ 7.29–7.41 (m,2H),6.71 (brs,2H,NH),4.22 (s,1H),3.73 (s,9H),2.51 (s,2H), 2.23 (m,1H),2.17 (m,1H),1.10 (s,3H),0.92 (s,3H); 13C NMR (75 MHz,CDCl3): δ 201.1,160.0,157.8,132.0,129.6,129.0, 119.1,107.2,106.4,60.3,59.3,57.1,50.0,38.0,32.3,28.9; GC–MS m/z 384 (M+); Elem. Anal. Calcd. for C21H24N2O5: C, 65.61; H,6.29; N,7.29; O,20.81; found: C,65.63; H,6.26; N, 7.31; O,20.83.
2-Amino-7,7-dimethyl-5-oxo-4-(2,3-dichlorophenyl)-5,6,7,8- tetrahydro-4H-chromene-3-carbonitrile (4i): White solid,mp 225–227°C,1H NMR (300 MHz,CDCl3): δ 7.17–7.21 (m,1H), 7.40–7.47 (m,1H),7.82–7.90 (m,1H),6.72 (brs,2H,NH),4.24 (s, 1H),2.61 (s,2H),2.28 (m,1H),2.06 (m,1H),0.94 (s,3H),0.74 (s, 3H); 13C NMR (75 MHz,CDCl3): δ 200.7,159.8,154.0,140.1,131.3, 130.2,130.0,119.2,113.2,67.6,60.0,44.3,41.2,32.1,28.6; GC–MS: m/z 363 (M+); Elem. Anal. Calcd. for C18H16Cl2N2O2: C,59.52; H, 4.44; Cl,19.52; N,7.71; O,8.81; found: C,59.53; H,4.43; Cl,19.55; N,7.69; O,8.83.
2-Amino-7,7-dimethyl-5-oxo-4-(pyridin-3-yl)-5,6,7,8-tetrahydro- 4H-chromene-3-carbonitrile (4m): Brownish solid; mp 187– 189 8C,1H NMR (300 MHz,CDCl3): δ 7.17–7.21 (m,1H),7.40–7.47 (m,1H),7.82–7.90 (m,1H),6.72 (brs,2H,NH),4.24 (s,1H),2.61 (s, 2H),2.28 (m,1H),2.06 (m,1H),0.94 (s,3H),0.74 (s,3H); 13C NMR (75 MHz,CDCl3): δ 200.0,161.3,154.0,153.6,142.8,135.1,129.9, 122.8,119.1,114.1,59.9,53.5,38.1,37.5,31.3,30.7; GC–MS: m/z 295 (M+); Elem. Anal. Calcd. for C17H17N3O2: C,69.14; H,5.80; N, 14.23; O,10.83; found: C,69.15; H,5.77; N,14.25; O,10.81. 2.2. Synthesis of solid aminopropylated SiO2
Silica gel was synthesized under acidic conditions. A known weight of sodium silicate solution (23.31 SiO2%; 7.48 Na2O%) was dilutedwith deionized water. 12% (w/v) SiO2 was added to 5.1mol/L H2SO4 solution under stirring with a peristaltic pump over 12.5 min at room temperature to adjust the SiO2 concentration to 8% (w/v),aged for 2 h,and then kept at 100°C for 12 h in a closed Simax glass bottle. The gel obtained was washed until it was sulfate free (BaCl2 test),dried at 100°C in an oven,and further calcined at 600°C for 6 h. Samples were cooled under vacuum and stored in a capped bottle over P2O5 in a desiccator. Surface functionalization of the silica gel was carried out by suspending the gel in a solution of 3-aminopropyltrimethoxysilane in dry toluene (solid:liquid,10:100,w/v) and refluxed at boiling temperature for 24 h. Samples were filtered,washed with isopropanol thoroughly,and dried at 100°C for overnight. Two different functionalized silica samples were prepared with APTMS using different APTMS: silica ratios (w/w),viz. 0.1 (0.56 mmol/g),which were designated as GN1. The synthesized material was characterized by powder X-ray diffraction and 29Si solid state NMR analysis.
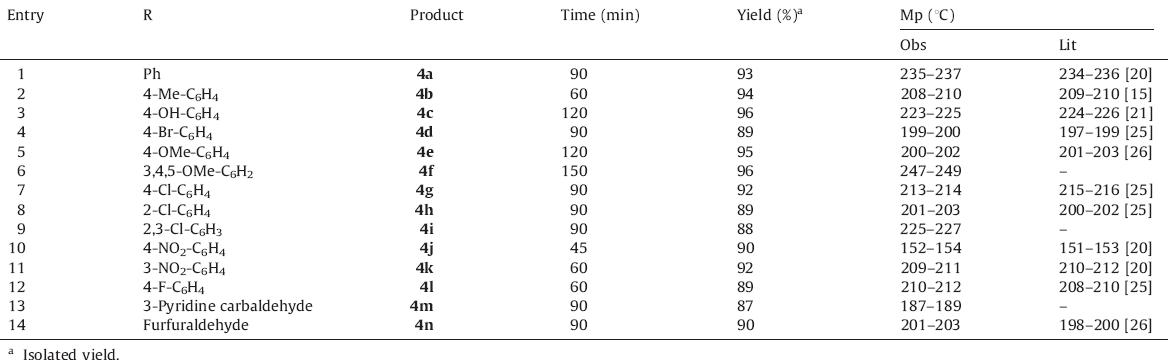
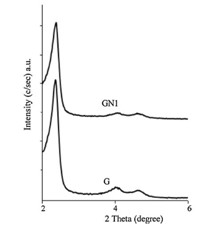
Powder X-ray diffraction: Powder X-ray diffraction (PXRD) patterns of functionalized silica showed the hexagonal structure of GN1; a typical mesoporous structure with three sharp peaks corresponding to the (1 0 0),(1 1 0),and (2 0 0) planes (Fig. 1). It has been seen that the value of d1 0 0 and a0 remained almost constant with the standard deviation of 0.35,indicating that the surface modification did not result in structural properties of GN1. 29Si NMR spectra: The 29Si NMR spectra of silica gel samples treated at different temperatures and functionalized silica gels are shown in Fig. 2. The three peaks at δ -112,-102 and -92 can be attributed to silicon in the siloxane binding environment without hydroxyl groups Q4[(SiO)4Si],isolated silanol groups Q3[(SiO)3Si–OH],and geminal silanol groups Q2[(SiO)2–Si– (OH)2] of the silica gel.
|
Download:
|
| Fig. 1.PXRD patterns of silica gel G and aminopropylated silica gel. | |
|
Download:
|
| Fig. 2.29Si MAS NMR spectra of silica gel and aminopropyl functionalized silica gels. | |
The presence of several free NH2 groups in the catalyst (such as GN3) [28] may lead to the formation of excess hydrogen bonding with acidic hydrogens of substrates such as phenols and hence may require prolonged time for completion of reaction. In the case of the GN 1 catalyst the probability of such interactions is less due to the presence of a single NH2 group. Hence,it catalyzes the reaction more efficiently in a short time to afford the corresponding products in high yields.
Preliminary efforts were mainly focused on the evaluation of different molar ratios of the catalyst AP-SiO2. Initially,the reaction was carried out using 5 mol% of the catalyst in ethanol; the product 4a was obtained in lower than 52% yield within 12 h. When the reaction was carried out using 10 mol% of catalyst,the reaction was completed in 5.5 h and the yield obtained of product 4a was 64%. Further increase in catalyst concentration did not show any significant effect on reaction time and yield of 4a. The reaction proceeds well in the presence of 10 mol% of the catalyst in ethanol at room temperature but require longer time for completion. As the temperature of reaction was raised slowly (Table 2); it was found that the reaction proceeds giving excellent yield at 70°C temperature within 1.5 h. Further increase in reaction temperature to 80°C showed similar results without change in yield or reaction time,and thus the optimized reaction conditions involve the use of 10 mol% of the catalyst at 70°C with a 1.5 h reaction time. Under the optimized reaction conditions,the ability of catalyst was explored in multicomponent reaction. Both electron-rich and electron deficient aryl aldehydes afforded 4H-chromene derivatives in 73%–98% yield in relatively short reaction times.
| Table 2 Effect of temperature on synthesis of 4a. |
Comparatively,the rate of reaction of electron-deficient aryl aldehyde was faster than the electron-rich aryl aldehyde. 4HChromene derivatives formed were obtained in pure form by recrystallization process. In order to extend the scope of amino propylated silica gel catalyzed multicomponent reactions,it was also successfully applied to aromatic heterocyclic aldehydes (Table 1).
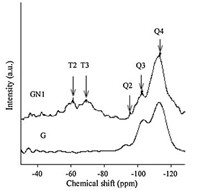
After performing the condensation of C–H activated ketones with aromatic aldehydes and alkylmalonates for the synthesis of 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4Hchromene- 3-carbonitrile derivatives under present conditions, ethyl acetate was added to the reaction mixture. The catalyst was separated by a simple filtration from the resulting heterogeneous mixture,washed with water 3–4 times,dried, and reused for consecutive runs under the same reaction conditions. The average isolated yield of 4a for five consecutive runs was 89%,which clearly demonstrates the practical reusability of this catalyst (Fig. 3). Structure of the recovered catalyst was also studied with XRD analysis. The powder XRD pattern of the recovered catalyst was similar to that of the fresh catalyst indicating that the recovered catalyst remains unaffected in its structure.
|
Download:
|
| Fig. 3.Reusability of solid aminopropylated SiO2 as a catalyst for the synthesis of 4a. | |
In summary,we synthesized silica gel supported amino functionalized heterogeneous solid aminopropylated SiO2 as a base catalyst. After structural characterization,its capability in organic transformations was investigated to synthesize 2-amino- 7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene- 3-carbonitrile 4a. The catalyst was found to catalyze the reaction under mild reaction conditions. The solid catalyst aminopropylated SiO2 was efficient for the synthesis of 4a and showed excellent stability and activity. The scope of the catalyst was further explored to catalyze the synthesis of 4H-chromene derivatives using different aromatic and heterocyclic aldehydes. The reactions proceeded well on all kind of aldehydes giving excellent yield of products in shorter reaction time. Finally,silica supported amine catalyst can be recovered by simple filtration procedure and reused without much loss in its activity. Acknowledgments
The authors are thankful to the Principal,DSM College,Parbhani and Principal,Deogiri College,Aurangabad for encouragement during the process of carrying out this work.
| [1] | G.J. Kelly, F. King, F. Kett, Waste elimination in condensation reactions of industrial importance, Green Chem. 4 (2002) 392-399. |
| [2] | J. Weitkamp, M. Hunger, U. Rymsa, Base catalysis on microporous and mesoporous materials: recent progress and perspectives, Microporous Mesoporous Mater. 48 (2001) 255-270. |
| [3] | R.A. Sheldon, Atom efficiency and catalysis in organic synthesis, Pure Appl. Chem. 72 (2000) 1233-1246. |
| [4] | A. Zapf, M. Beller, Fine chemical synthesis with homogeneous palladium catalysts: examples, status and trends, Top. Catal. 19 (2002) 101-109. |
| [5] | B.M. Choudary, M.L. Kantam, P. Sreekanth, et al., Knoevenagel and Aldol condensations catalysed by a new diamino-functionalised mesoporous material, J. Mol. Catal. A 142 (1999) 361-365. |
| [6] | Y. Kubota, Y. Nishizaki, H. Ikeya, et al., Organic silicate hybrid catalysts based on various defined structures for Knoevenagel condensation, Microporous Mesoporous Mater. 70 (2004) 135-149. |
| [7] | Y. Wan, D. Zhao, On the controllable soft-templating approach to mesoporous silicates, Chem. Rev. 107 (2007) 2821-2860. |
| [8] | Q.Q. Wang, D.F. Shantz, Ordered mesoporous silica-based inorganic nanocomposites, J. Solid State Chem. 181 (2008) 1659-1669. |
| [9] | X.G. Wang, K.S.K. Lin, J.C.C. Chan, S.F. Cheng, Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials, J. Phys. Chem. B 109 (2005) 1763-1769. |
| [10] | Y. Kubota, K. Goto, S. Miyata, et al., Enhanced effect of mesoporous silica on basecatalyzed Aldol reaction, Chem. Lett. 32 (2003) 234-235. |
| [11] | A.C. Blanc, D.J. Macquarrie, S. Valle, et al., The preparation and use of novel immobilised guanidine catalysts in base-catalysed epoxidation and condensation reactions, Green Chem. 2 (2000) 283-288. |
| [12] | X.G. Wang, Y.H. Tseng, J.C.C. Chan, S.F. Cheng, Catalytic applications of aminopropylated mesoporous silica prepared by a template-free route in flavanones, J. Catal. 233 (2005) 266-275. |
| [13] | M. Luechinger, A. Kienhofer, G.D. Pirngruber, Immobilized complexes of metals with amino acid ligands -a first step toward the development of new biomimetic catalysts, Chem. Mater. 18 (2006) 1330-1336. |
| [14] | D. Lu, Y. Li, Y. Gong, Organocatalytic asymmetric tandem Michael additionhemiacetalization: a route to chiral dihydrocoumarins, chromanes, and 4Hchromenes, J. Org. Chem. 75 (2010) 6900-6907. |
| [15] | W. Kemnitzer, J. Drewe, S. Jiang, et al., Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell and caspase-based high throughput screening assay. 4. Structure-activity relationships of N-alkyl substituted pyrrole fused at the 7,8-positions, J. Med. Chem. 51 (2008) 417-423. |
| [16] | E.C. Witte, P. Neubert, A. Roesch, 7-(Piperazinyl propoxy)-2H-1-benzopyran-2-ones, Ger. Offen DE, 3427985, Chem. Abstr. 104 (1986) 224915f. |
| [17] | J. Kuthan, Pyrans, thiopyrans, and selenopyrans, Adv. Heterocycl. Chem. 34 (1983) 145-157. |
| [18] | M. Suarez, E. Salfran, Y. Verdecia, et al., X-ray and theoretical structural study of novel 5,6,7,8-tetrahydrobenzo-4H-pyrans, Tetrahedron 58 (2002) 953-960. |
| [19] | A. Molla, E. Hossain, S. Hussain, Multicomponent domino reactions: borax catalyzed synthesis of highly functionalised pyran-annulated heterocycles, RSC Adv. 3 (2013) 21517-21523. |
| [20] | K. Niknam, A. Jamali, Silica-bonded N-propylpiperazine sodium n-propionate as recyclable basic catalyst for synthesis of 3,4-dihydropyrano[c]chromene derivatives and biscoumarins, Chin. J. Catal. 33 (2012) 1840-1849. |
| [21] | S. Paul, P. Bhattacharyya, A.R. Das, One-pot synthesis of dihydropyrano[2,3-c]chromenes via a three component coupling of aromatic aldehydes, malononitrile, and 3-hydroxycoumarin catalyzed by nano-structured ZnO in water: a green protocol, Tetrahedron Lett. 52 (2011) 4636-4641. |
| [22] | N. Martin, C. Pascual, C. Seoane, J.L. Soto, The use of some activated nitriles in heterocyclic syntheses, Heterocycles 26 (1987) 2811-2816. |
| [23] | A.F. Harb, A.M. Hesien, S.A. Metwally, M.H. Elnagdi, The reaction of ethyl 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylate with nucleophilic reagents, Ann. Chem. (1989) 585-590. |
| [24] | S.E. Zayed, E.I. Abou-Elmaged, S.A. Metwally, M.H. Elnagdi, Reactions of sixmembered heterocyclic β-enaminonitriles with electrophilic reagents, Chem. Commun. 56 (1991) 2175-2182. |
| [25] | I. Devi, P.J. Bhuyan, Sodium bromide catalysed one-pot synthesis of tetrahydrobenzo[b]pyrans via a three-component cyclocondensation under microwave irradiation and solvent free conditions, Tetrahedron Lett. 45 (2004) 8625-8627. |
| [26] | X.S. Wang, D.Q. Shi, S.J. Tu, C.S. Yao, A convenient synthesis of 5-oxo-5,6,7,8-tetrahydro-4H-benzo-[b]-pyran derivatives catalyzed by KF-alumina, Syn. Commun. 33 (2003) 119-126. |
| [27] | V. Manu, H.M. Mody, H.C. Bajaj, R.V. Jasra, Adsorption of Cu2+ on amino functionalized silica gel with different loading, Ind. Eng. Chem. Res. 48 (2009) 8954-8960. |
| [28] | R.L. Magar, P.B. Thorat, V.B. Jadhav, et al., Silica gel supported polyamine: a versatile catalyst for one pot synthesis of 2-amino-4H-chromene derivatives, J. Mol. Catal. A: Chem. 374-375 (2013) 118-124. |