Heterocyclic compounds are important and valuable parts of biologically active molecules and natural products; therefore,the design of new strategies to synthesize them is currently an important area of research. An interesting class of these compounds is 1,2,4-triazolidine-3,5-diones,which are five-membered heterocyclic compounds including three azo atoms with a wide variety of aliphatic as well as aromatic constituents at position 4 [1]. 1,2,4-Triazolidine-3,5-diones are of interest because of their role as reagents in laboratory and industry; for instance, application in preparing of automobile air bags,as a blowing agent in plastic compounds,in the production of herbicides,as an anticonvulsant,in antifungal compounds,and in polymeric materials [2, 3, 4, 5, 6]. These compounds are also used for the preparation of organometallic compounds [7]. There are few reports for the synthesis of these heterocyclic compounds [1, 8, 9]. Many of these reported methods suffer from one or more drawbacks,such as low yields,hazardous reaction conditions,and multistep processes. Therefore,the search for a more suitable preparation of 1,2,4- triazolidine-3,5-diones continues today. 2. Experimental
Typical procedure for the synthesis of 4-substituted-urazoles: ptoluidine (3mmol) and cesium carbonate (3.5mmol) were dissolved in anhydrous 1,4-dioxane (10 mL). Triphosgene (1mmol) was added in portions over 2,3 min,and this mixture was stirred at room temperature. After 1.5 h,ethyl carbazate (3.2mmol) was added,and the reaction mixture was stirred overnight. Following evaporation to dryness,the reaction mixture was refluxed in aqueous 5 mol/L KOH for 5 h then cooled down in an ice bath. The solutionwas neutralized with concentrated HCl to reach pH1,2. The white crystalline product was collected and dried to give 4-(4- methylphenyl)-1,2,4-triazolidine-3,5-dione with 84% yield.
4-(4-Isopropylphenyl)-1,2,4-triazolidine-3,5-dione (4a): White crystalline solid,0.162 g (74%); mp: 231–234°C; 1H NMR (400 MHz,DMSO-d6): δ 10.45 (s,2H),7.36 (br,4H),2.94 (septet, 1H,J = 6.8 Hz),1.21 (d,6H,J = 6.8 Hz); 13C NMR (100.6 MHz, DMSO-d6): δ 154.1,148.5,130.0,127.1,126.6,33.7,24.3. Anal. Calcd. for C11H13N3O2: C,60.26; H,5.98; N,19.17. Found: C,61.13; H,5.68; N,18.89.
4-(4-Bromophenyl)-1,2,4-triazolidine-3,5-dione (4c): White crystalline solid,0.159 g (62%); mp: 210–212°C; 1H NMR (400 MHz,DMSO-d6): δ 10.60 (s,2H),7.70 (d,2H,J = 8.8 Hz), 7.47 (d,J = 8.8 Hz,2H); 13C NMR (100.6 MHz,DMSO-d6): δ 153.4, 132.2,131.8,128.2,120.7. Anal. Calcd. for C8H6BrN3O2: C,37.53; H, 2.36; N,16.41. Found: C,37.88; H,2.68; N,15.87. 4-(4-Ethylphenyl)-1,2,4-triazolidine-3,5-dione (4d): White crystalline solid,0.201 g (98%); mp: 246–248°C; 1H NMR(400 MHz,DMSO-d6): δ 10.46 (s,2H),7.33–7.36 (m,4H),2.65 (q, 2H,J = 7.2 Hz),1.20 (t,3H,J = 7.2 Hz); 13C NMR (100.6 MHz,DMSOd6): d 154.0,143.9,129.9,128.6,126.6,28.3,16.1. Anal. Calcd. for C10H11N3O2: C,58.53; H,5.40; N,20.48. Found: C,58.34; H,5.11; N, 20.21.
4-(2,4-Dimethoxyphenyl)-1,2,4-triazolidine-3,5-dione (4e): White crystalline solid,0.130 g (55%); mp: 245–246°C; 1H NMR (400 MHz,DMSO-d6): δ 10.20 (s,2H),7.16 (d,1H,J = 8.4 Hz),6.71 (s,1H),6.60 (d,1H,J = 8.4 Hz),3.82 (s,3H),3.76 (s,3H); 13C NMR (100.6 MHz,DMSO-d6): δ 161.6,156.9,154.8,131.4,113,2,105.4, 99.7,56.3,56.0. Anal. Calcd. for C10H11N3O4: C,50.63; H,4.67; N, 17.71. Found: C,49.28; H,3.13; N,17.78.
4-(4-Tritylphenyl)-1,2,4-triazolidine-3,5-dione (4f): White crystalline solid,0.411 g (98%); mp: 300°C (dec.); 1H NMR (400 MHz,DMSO-d6): δ 10.58 (s,2H),7.02–7.41 (m,19H); 13C NMR (100.6 MHz,DMSO-d6): δ 153.7,146.7,146.2,131.2,130.9, 130.1,128.4,126.6,125.6,64.8. Anal. Calcd. for C27H21N3O4: C, 77.31; H,5.05; N,10.02. Found: C,76.89; H,2.90; N,9.52.
4-(4-Fluorophenyl)-1,2,4-triazolidine-3,5-dione (4h): White crystalline solid,0.150 g (77%); mp: 269–270°C; 1H NMR (400 MHz,DMSO-d6): δ 10.53 (s,2H),7.45-7.53 (m,2H),7.31– 7.36 (m,2H); 13C NMR (100.6 MHz,DMSO-d6): δ 161.4 (d,Jc– F = 243 Hz),153.8,128.7 (d,Jc–F = 9 Hz),120.5 (d,Jc–F = 8 Hz),116.1 (d,Jc–F = 23 Hz). Anal. Calcd. for C8H6FN3O4: C,49.24; H,3.10; N, 21.53. Found: C,49.97; H,2.56; N,21.53.
The NMR spectra can be found in the Supporting information file. 3. Results and discussion
In light of the aforementioned biologic,laboratorial,and industrial activities and as part of our ongoing program towards the synthesis of heterocyclic compounds [10, 11, 12, 13, 14, 15],we delineated an efficient route for the synthesis of urazole derivatives.
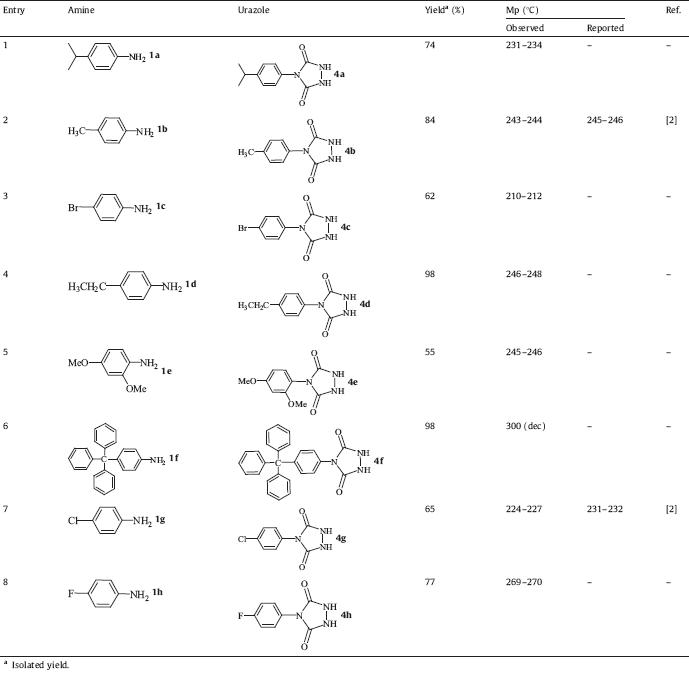
Initially,we performed the reaction between 4-isopropylaniline 1a and triphosgene in the presence of different bases (such as triethylamine,potassium hydroxide,sodium carbonate,and cesium carbonate) and solvents (such as dichloromethane, acetone,1,4-dioxane,ethyl acetate,and tetrahydro furane) to achieve 4-isopropylisocyanate 2a. After consumption of 4-isopropylaniline, ethyl carbazate was added to the solution to afford intermediate 3a. The best solvent and base for these two steps were 1,4-dioxane and cesium carbonate,respectively. After consumption of 4-isopropylisocyanate,the solvent was evaporated and 5 mol/L KOH was added to the mixture and refluxed; this step was found to be completed within 5 h,affording 4-(4-isopropylphenyl)- 1,2,4-triazolidine-3,5-dione 4a (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of 4-(4-isopropylphenyl)-1,2,4-triazolidine-3,5-dione. | |
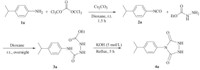
Based on these results,we investigate the scope and limitations of this synthesis strategy by varying the structure of amine. Therefore,a novel series of 4-substituted urazoles has been synthesized by a one-pot multicomponent reaction using different types of anilines,triphosgene,and ethyl carbazate (Scheme 2).

|
Download:
|
| Scheme 2.Synthesis of 4-substituted-1,2,4-triazolidin-3,5-diones. | |
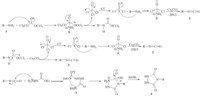
The results of these syntheses are summarized in Table 1. A plausible mechanism for the synthesis of 4-substituted urazoles, has been outlined in Scheme 3,based on previously reported papers [1, 8, 9, 16]. The interesting point of this mechanism is the in situ generation of three equivalent isocyanates (2) from the reaction of one equivalent triphosgene and three equivalent aniline. Subsequently,reaction of isocyanate with ethyl carbazate was followed by cyclization of intermediate (3) to generate corresponding 4-substituted-1,2,4-triazolidin-3,5-dione (4).
| Table 1 Synthesis of 4-substituted-1,2,4-triazolidin-3,5-diones via one-pot combination of different types of anilines with triphosgene and ethyl carbazate in the presence of cesium carbonate. |
|
Download:
|
| Scheme 3.Mechanism of the urazole synthesis. | |
In summary,we have delineated an efficient synthesis of structurally diverse 4-substituted urazoles via one-pot combination of a variety of anilines with triphosgene and ethyl carbazate in the presence of cesium carbonate. Simple reaction conditions, broad substrate scope,and no additional solvent extraction steps are the advantages of this procedure. The greenness and simplicity of this synthetic procedure makes it an interesting alternative to other approaches. Acknowledgment
Financial support to this work by the Ilam University,Ilam,Iran is gratefully acknowledged. Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet. 2013.11.020.
| [1] | S. Mallakpour, Z. Rafiee, A novel one-pot synthesis of 4-substituted 1,2,4-triazolidine-3,5-diones, Synlett (2007) 1255-1256. |
| [2] | R. Simlot, R.A. Izydore, O.T. Wong, A.H. Hall, Hypolipidemic activity of 4-substituted 1,2-diacyl-1,2,4-triazolidine-3,5-diones in rodents, J. Pharm. Sci. 83 (1994) 367-371. |
| [3] | V. Kanagarajan, J. Thanusu, M.R. Ezhilarasi, M. Gopalakrishnan, Solvent-free one pot synthesis, characterization, antibacterial and antifungal activities of novel spiropiperidinyl-1,2,4-triazolidine-3-thiones, Chem. Heterocycl. Compds. 47 (2011) 60-66. |
| [4] | S. Mallakpour, Z. Rafiee, Ionic liquids as novel and green media for clean synthesis of soluble aromatic-aliphatic poly(amide-ester)s containing hydroxynaphthalene urazole moiety, Polym. Adv. Technol. 19 (2008) 1015-1023. |
| [5] | S. Mallakpour, Z. Rafiee, Solid-state polymerization of 4-(4-dimethylaminophenyl)-urazole with diisocyanates, Polym. Bull. 60 (2008) 507-514. |
| [6] | S. Mallakpour, Z. Rafiee, Efficient combination of ionic liquids and microwave irradiation as a green protocol for polycondensation of 4-(3-hydroxynaphthalene) 1,2,4-triazolidine-3,5-dionewith diisocyanates, Polymer48(2007) 5530-5540. |
| [7] | D.A. Gianolio, M. Lanfranchi, F. Lusardi, L. Marchio, M.A. Pellinghelli, Synthesis and characterization of Co(Ⅱ), Ni(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) complexes of 4-amino-1,2,4-triazolidine-3,5-dione (urazine), Inorg. Chim. Acta 309 (2000) 91-102. |
| [8] | S. Mallakpour, Z. Rafiee, Novel and efficient synthesis of 4-substituted-1,2,4-triazolidine-3,5-diones from anilines, Synth. Commun. 37 (2007) 1927-1934. |
| [9] | K.H. Park, L.J. Cox, Solid-phase synthesis of 1,2,4-triazolidine-3,5-diones, Tetrahedron Lett. 43 (2002) 3899-3901. |
| [10] | A. Ghorbani-Choghamarani, M.A. Zolfigol, P. Salehi, et al., An efficient procedure for the synthesis of Hantzsch 1,4-dihydropyridines under mild conditions, Acta Chim. Slov. 55 (2008) 644-647. |
| [11] | A. Ghorbani-Choghamarani, M. Hajjami, H. Goudarziafshar, et al., Catalytic oxidation of urazoles and bisurazoles to their corresponding triazolinediones using aluminium nitrate and a catalytic amount of silica sulfuric acid, Monatsh. Chem. 140 (2009) 607-610. |
| [12] | A. Ghorbani-Choghamarani, M.A. Zolfigol, M. Hajjami, et al., Nano aluminium nitride as a solid source of ammonia for the preparation of Hantzsch 1,4-dihydropyridines and bis-(1,4-dihydropyridines) in water via one pot multicomponent reaction, J. Braz. Chem. Soc. 22 (2011) 525-531. |
| [13] | A. Ghorbani-Choghamarani, Z. Chenani, S. Mallakpour, Supported nitric acid on silica gel and polyvinyl pyrrolidone (PVP) as an efficient oxidizing agent for the oxidation of urazoles and bis-urazoles, Synth. Commun. 39 (2009) 4264-4270. |
| [14] | A. Ghorbani-Choghamarani, M. Hajjami, M. Norouzi, et al., Diastereoselective and one-pot synthesis of trans-isoquinolonic acids via three-component condensation of homophthalic anhydride, aldehydes, and ammonium acetate catalyzed by aspartic acid, Tetrahedron 69 (2013) 6541-6544. |
| [15] | A. Ghorbani-Choghamarani, P. Zamani, Three component reactions: an efficient and green synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and thiones using silica gel-supported l-pyrrolidine-2-carboxylic acid-4-hydrogen sulfate, Chin. Chem. Lett. 69 (2013) 804-808. |
| [16] | Y.C. Charalambides, S.C. Moratti, Comparison of base-promoted and self-catalyzed conditions in the synthesis of isocyanates from amines using triphosgene, Synth. Commun. 37 (2007) 1037-1044. |