Hollow microspheres have attracted much attention because of their special characteristics,such as low density,low thermal conductivity,high specific surface and good flow ability [1]. So far, various porous microspheres with unique characteristics such as hollow spheres with a single cavity [2, 3, 4],hollow spheres with multiple shells [5, 6],and hollow sphere with multicavities [7], have been fabricated by the templating method,emulsion processing,high temperature smelting,layer-by-layer self-assembly techniques,etc. Wherein,hollow spheres with multicavities as an important class of hollow sphere have gained growing attention for their potential applications such as delivery systems,adsorption and catalysis [8, 9, 10]. Although organic spheres with multicavities consisting of diverse polymers have been prepared [11- 13],manipulation of inorganic spherical particles with multicavities is still a challenging task. So far,cage-like SiO2 hollow spheres have been prepared by templating methods and templatefree methods in emulsion route [14, 15, 16]. However,we notice that the preparation of metal oxide hollow spheres with multicativies including titania (TiO2),zirconia (ZrO2) and alumina (Al2O3) hollow spheres have rarely been mentioned in the literature. To the best of our knowledge,there is only one paper concerning the transitionmetal oxide microspheres with multicavities,which were prepared by using template in aerosol-assisted self-assembly method [17]. However,the reported method to prepare transition-metal oxide microspheres with multicavities needs heat treatment to remove the latex template.
In the present work,we demonstrate a facile one-step synthesis of cage-like porous titania microspheres via oil/water (O/W) emulsion accompanied by a sol–gel reaction without any templates. The presence of polyvinylpyrrolidone (PVP) played an important role in forming porous structure of titania hollow microspheres with multicavities,while Span 80 had great effects on preserving spherical structure of both macrocavities and TiO2 particles. Moreover,zirconia and alumina hollow spheres with multicavities were also fabricated via the same method mentioned. It is anticipated that the method presented in this work will offer an approach to fabricate metal oxides hollow spheres with multicavities,including their composites. 2. Experimental 2.1. Materials
1-Octanol,ethyl acetoacetate (EAA,AR),zirconium propoxide (70 wt.% of popanol solution) and aluminum tri-sec-butoxide (97%) were purchased from Aladdin Reagent Co.,Ltd. Other chemicals, i.e.,tetrabutyl titanate (TBT,AR),Span 80 (CP),sodium dodecyl sulfate (SDS,CP),alkylphenol ethoxylates (OP-10,CP) and polyvinylpyrrolidone (PVP,K30,CP) were purchased from Sinopharm Chemical Reagent Co. Ltd. All the chemicals were used as received. 2.2. Synthesis
The detailed preparation of the gel microspheres was as follows. First,an external water phase was prepared by adding 4.0 g of SDS and 8.0 g of OP-10 in 300 g of deionized water under strong stirring at 1000 rpm. Second,2.377 g of EAA and 6.217 g of TBT were added to 10.450 g of 1-octanol with constant stirring for 1 h to prepare the oil phase. Then,a certain amount (0.190,0.571,1.142 and 1.903 g) of PVP and 0.571 g of Span 80 were added to the oil phase under stirring for 1 h as an additive. Third,the mentioned oil phase was added to the external water phase once under strong stirring at 1000 rpm. After stirring for 24 h,the samples were collected by centrifuge and dried at 40°C for 24 h.
The fabrication of zirconia and alumina microspheres followed the same process,except with zirconium propoxide and aluminum tri-sec-butoxide as the precursors,respectively. 2.3. Characterization
The microstructures including average cavity sizes and size distributions of the TiO2 microspheres were performed using scanning electronic microscope (SEM,FEI Siron). Thermal analysis for the TiO2 gel microspheres was carried out using a DTA analyzer (German Netzsch Co. STA449C) from room temperature to 700°C in air at a heating rate of 10°C/min. 3. Results and discussion
It was found that the initial oil phase was a yellow transparent solution. When the oil phase was mixed with water phase and broken into small droplets by high-speed shearing,water molecules around the oil droplets diffused through oil–water interface and reacted with TBT to initiate the sol–gel process. The sol–gel transition of oil droplets,which built the solid spheres,is caused by two kinds of reaction,i.e.,hydrolysis and polycondensation [18]. The effects of PVP will be discussed and a preliminary mechanism will be suggested accordingly.
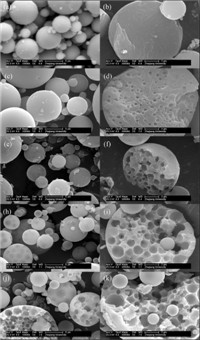
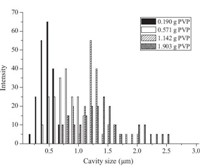
Fig. 1 shows the SEM images of TiO2 microspheres fabricated with different amounts of PVP. Spherical structures are obtained for all five particles,and the surfaces of the microspheres fabricated with different amounts of PVP are generally smooth. However,different interior structures are observed for the five samples. The microspheres fabricated without PVP are solid as shown in Fig. 1b,while the addition of PVP led to cage-like porous structures as shown in Fig. 1b,d,f,i,k. The average cavity diameters calculated from the SEM images are ~500 nm,~750 nm, ~1.2 mm,and~1.3 mm,respectively. In addition,the microspheres obtained with 1.903 g of PVP show generally broken structures, which can be attributed to the fragility caused by the decrease in wall thickness of cavities. As shown in Fig. 2,the cavity size distributions also become wider with the increase of PVP content generally.
|
Download:
|
| Fig. 1.SEM images of the titania microsphere synthesized with 0 (a and b),0.190 (c and d),0.571 (e and f),1.142 (h and i),and 1.903 g (j and k) PVP. | |
|
Download:
|
| Fig. 2.Cavity size distribution of the microspheres fabricated with different PVP contents. | |
To clarify the role of PVP in fabricating the cage-like porous structure,DTA measurements were performed for the TiO2 microspheres (Fig. 3a) and the supernatant phases obtained by centrifuge (Fig. 3b),respectively. For the supernatant phase,two exothermic peaks at around 430 and 500°C are observed,which are attributed to the decomposition of PVP. For the TiO2 microspheres, only an exothermic peak at 270°C is observed for all five samples,which can be associated with the decomposition of butoxy and residual 1-octanol [19]. As shown by the DTA measurement,PVP is preferentially distributed into the water phase,rather than the titania oligomers rich phase. It indicates that the interaction between polymerizing titania oligomers and PVP is less attractive,although the reported literature mentioned that there is a strong interaction between PVP and Ti atom [20, 21]. The decreasing interaction is probably attributed to the decreased polarity of titania oligomers and the increased steric hindrance induced by polymerization [22].

|
Download:
|
| Fig. 3.DTA curves of titania microspheres fabricated with different PVP contents (a),and the supernatant phases obtained by centrifuge (b). | |
The mechanism for the formation of the hollow spheres with multicavities may be explained on the basis of nucleation-growth phase-separation model,which is schematically shown in Fig. 4. As described by Nakanishi [18],the polymerization and gel forming reaction are thermodynamically analogous to the continuous cooling of a glass-forming liquid into a miscibility gap,which can be described as ‘‘chemical cooling’’. An initial single-phase solution containing a polymerizable component becomes less stable with an increase in the molecular weight of the component,and results in the separation into different phases. The phase-separated domains go through a coarsening process induced by additional molecules diffusing toward the nuclei. Also,the sol–gel transition can freeze the phase separation structures to prevent further coarsening of the separated domains. In this system in the presence of PVP,the increasing molecular weight of monomer- TiO2 polymer via the sol–gel process could cause the system to be immiscible and drive the phase separation. During the sol–gel process,the PVP will precipitate inside the oil phase and become the nucleation center. Then the ethyl acetoacetate released diffuses toward the center to induce coarsening process. The sol–gel transition finally freezes the phase separation structures to prevent further coarsening. The increase of PVP may enhance the phase-separation tendency,which can cause an early onset of phase separation relative to the sol–gel transition [23],and results in a relative long time for the coarsening process to build an enlarged pore size. Moreover,the PVP was almost dissolved in the water phase as characterized by the DTA measurement,which might indicate the diffusion of PVP and ethyl acetoacetate into the water phase due to their good water solubility.

|
Download:
|
| Fig. 4.Illustrative mechanism for the formation of hollow microspheres with multicavities. | |
The sol–gel process inside the oil phase was greatly influenced by the diffusion of water molecules. Thus the polarity of the solvent used in the oil phase might have great effects on the porous structure of the microspheres. In order to confirm the effect of the polarity of the solvent used in the oil phase,20 wt.% 1-octanol was replaced by n-propanol and atoleine,respectively. Fig. 5 shows the SEM images of the microspheres fabricated with 20 wt.% replacement of 1-octanol. As shown,the microspheres fabricated in the presence of n-propanol (Fig. 5a and b) have smaller pore diameters (~550 nm) than the microspheres without solvent replacement (Fig. 1e and f),while the microspheres fabricated in the presence of atoleine (Fig. 5c and d) possess lower pore density. Herein,we may suggest that the addition of polar solvent to the oil phase facilitates the diffusion of water in the oil phase and accelerates the sol–gel process. As mentioned,the polymerization and gel forming reaction are thermodynamically analogous to the continuous cooling of a glass-forming liquid into a miscibility gap. The accelerated sol–gel process may result in high degree of ‘‘chemical supercooling’’,which leads to a relatively high nucleation rate in the course of the phase separation. Thus we can observe that the microspheres fabricated with n-propanol additive contain higher pore density and smaller pore diameters. On the contrary,the atoleine additive may suppress the sol–gel process by retarding the diffusion of water molecule. Thus the lower pore density can be attributed to the relatively low nucleation rate in the course of the phase separation.

|
Download:
|
| Fig. 5.Illustrative mechanism for the formation of hollow microspheres with multicavities. | |
In addition,the Span 80 additive also plays an important role on the microstructure of microspheres. It is expected that Span 80 molecules can locate on the interface of the separated domains to act as a stabilizer so that the separated domains could experience less deformation during the coarsening process. As shown in Fig. 6, the absence of Span 80 will result in a coarse surface and irregular inner cavities.

|
Download:
|
| Fig. 6.SEM images of the TiO2 microspheres fabricated with 0.571 g PVP and without Span 80. | |
In the literature,porous structures can also be fabricated via W/ O/W emulsions [11, 12, 24]. In order to confirm the phase separation mechanism,we prepared TiO2 gel film by spreading the oil phase containing 0.571 g of PVP and 0.571 g of Span 80 on water surface as depicted in Fig. 7a. The diffused water initiated the sol–gel process of the oil phase,which produced a TiO2 gel film. Fig. 7b and c shows the SEM images of the TiO2 gel film and the cross section,respectively. It is observed that the TiO2 gel film obtained without vigorous stirring also has a porous structure consisting of incontinuous cavities,which further demonstrated that the porous structure of the TiO2 microspheres should not be attributed to W/O/W emulsion [25].

|
Download:
|
| Fig. 7.Schematic picture and SEM images of the prepared titania gel film. | |

|
Download:
|
| Fig. 8.SEM images of zirconia (a and b) and alumina (c and d) microspheres with incontinuous multicavities. | |
A novel method was proposed to fabricate titania hollow microspheres with incontinuous multicavities. The titania hollow microspheres with incontinuous multicavities were fabricated through a one-step process without templates,where O/W emulsion process accompanied by a sol–gel reaction was employed effectively. The addition of PVP to the oil phase changed the inner structure of the obtained microspheres to form incontinuous cavities in nanometer or micrometer scale,while the microspheres obtained in the absence of PVP were solid. The addition of n-propanol and atoleine,which altered the polarity of the oil phase,was proven to have great effect on the interior structures including the change of cavity sizes and pore density. The Span 80 was added as a stabilizer for preserving the spherical shape,and the absence of Span 80 in the oil phase resulted in a coarse pseudo-spherical shape and irregular inner cavities. The preliminary theory based on phase-separation was advanced to illustrate the mechanism for the cavity formation,which was different from the mechanism based on W/O/W emulsion. This method was also used to fabricate zirconia and alumina microspheres with incontinuous multicavities. Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 51372225) and Zhejiang Provincial Natural Science Foundation of China (No. LY13B010001).
| [1] | J. Hu, M. Chen, X.S. Fang, L.M. Wu, Fabrication and application of inorganic hollow spheres, Chem. Soc. Rev. 40 (2011) 5472-5491. |
| [2] | S. Schacht, Q. Huo, I.G. Voigt-Martin, G.D. Stucky, F. Schü th, Oil-water interface templating of mesoporous macroscale structures, Science 273 (1996) 768-771. |
| [3] | J.H. Park, C. Oh, S. Shin, S.K. Moon, S.G. Oh, Preparation of hollowsilicamicrospheres in w/o emulsions with polymers, J. Colloid Interface Sci. 266 (2003) 107-114. |
| [4] | T. Nakashima, N. Kimizuka, Interfacial synthesis of hollow TiO2 microspheres in ionic liquids, J. Am. Chem. Soc. 125 (2003) 6386-6387. |
| [5] | H.L. Xu, W.Z. Wang, Template synthesis of multishelled Cu2O hollow spheres with a single-crystalline shell wall, Angew. Chem. Int. Ed. 46 (2007) 1489-1492. |
| [6] | J.G. Guan, F.Z. Mou, Z.G. Sun, W.D. Shi, Preparation of hollow spheres with controllable interior structures by heterogeneous contraction, Chem. Commun. 46 (2010) 6605-6607. |
| [7] | Y.B. Kim, K.S. Yoon, A physical method of fabricating hollow polymer spheres directly from oil/water emulsions of solutions of polymers, Macromol. Rapid Commun. 25 (2004) 1643-1649. |
| [8] | E. Kamio, S. Yonemura, T. Ono, H. Yoshizawa, Microcapsules with macroholes prepared by the competitive adsorption of surfactants on emulsion droplet surfaces, Langmuir 24 (2008) 13287-13298. |
| [9] | J. Han, P. Fang, J. Dai, R. Guo, One-pot surfactantless route to polyaniline hollow nanospheres with incontinuous multicavities and application for the removal of lead ions from water, Langmuir 28 (2012) 6468-6475. |
| [10] | J.X. Xu, G.J. Chen, R. Yan, et al., One-stage synthesis of cagelike porous polymeric microspheres and application as catalyst scaffold of Pd nanoparticles, Macromolecules 44 (2011) 3730-3738. |
| [11] | H. Zhang, A.I. Cooper, Synthesis of monodisperse emulsion-templated polymer beads by oil-in-water-in-oil (O/W/O) sedimentation polymerization, Chem. Mater. 14 (2002) 4017-4020. |
| [12] | H. Wang, M.Z. Wang, X.W. Ge, One-step fabrication of multihollow polystyrene particles from miniemulsion system with nonionic surfactant, Polymer 49 (2008) 4974-4980. |
| [13] | G.H. Zhang, R.X. Hou, D.X. Zhan, et al., Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility, Chin. Chem. Lett. 24 (2013) 710-714. |
| [14] | L. Li, E.S.G. Choo, X.S. Tang, J. Ding, J.M. Xue, A facile one-step route to synthesize cage-like silica hollow spheres loaded with superparamagnetic iron oxide nanoparticles in their shells, Chem. Commun. 45 (2009) 938-940. |
| [15] | F. Iskandar, K. Okuyama, Controllability of pore size and porosity on self-organized porous silica particles, Nano Lett. 2 (2002) 389-392. |
| [16] | X. Du, J.H. He, Fine-tuning of silica nanosphere structure by simple regulation of the volume ratio of cosolvents, Langmuir 26 (2010) 10057-10062. |
| [17] | D. Grosso, G.J. de A.A. Soler-Illia, E.L. Crepaldi, B. Charleux, C. Sanchez, Nanocrystalline transition-metal oxide spheres with controlled multi-scale porosity, Adv. Funct. Mater. 13 (2003) 37-42. |
| [18] | K. Nakanishi, Pore structure control of silica gels based on phase separation, J. Porous Mater. 4 (1994) 67-112. |
| [19] | Q.L. Zhang, F. Wu, H. Yang, D. Zou, Preparation and dielectric properties of (Ca0.61,Nd0.26)TiO3 nanoparticles by a sol-gel method, J. Mater. Chem. 18 (2008) 5339-5343. |
| [20] | T. Sato, A. Sato, T. Arai, Adsorption of polyvinylpyrrolidone on titanium dioxide from binary solvents (methanol/water) and its effect on dispersion stability, Colloids Surf. A 142 (1998) 117-120. |
| [21] | M.P. Zheng, M.Y. Gu, Y.P. Jin, G.L. Jin, Preparation, structure and properties of TiO2-PVP hybrid films, Mater. Sci. Eng. B 77 (2000) 55-59. |
| [22] | Y. Frere, P. Gramain, Reaction kinetics of polymer substituents: macromolecular steric hindrance effect in quaternization of poly(vinylpyridines), Macromolecules 25 (1992) 3184-3189. |
| [23] | K. Nakanishi, N. Tanaka, Sol-gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations, Acc. Chem. Res. 40 (2007) 863-873. |
| [24] | F. Gao, Z.G. Su, P. Wang, G.H. Ma, Double emulsion templated microcapsules with single hollow cavities and thickness-controllable shells, Langmuir 25 (2009) 3832-3838. |
| [25] | A.Y. Khan, S. Talegaonkar, Z. Iqbal, J.F. Ahmed, R.K. Khar, Multiple emulsions: an overview, Curr. Drug Deliv. 3 (2006) 429-443. |
| [26] | Y. Tokudome, K. Fujita, K. Nakanishi, K. Miura, K. Hirao, Synthesis of monolithic Al2O3 with well-defined macropores and mesostructured skeletons via the sol-gel process accompanied by phase separation, Chem. Mater. 19 (2007) 3393-3398. |
| [27] | F.Q. Lin, W.S. Dong, C.L. Liu, Z.T. Liu, M.Y. Li, In situ source-template-interface reaction route to hollow ZrO2 microspheres with mesoporous shells, J. Colloid Interface Sci. 323 (2008) 365-371. |
| [28] | J. Konishi, K. Fujita, S. Oiwa, K. Nakanishi, K. Hirao, Crystalline ZrO2 monoliths with well-defined macropores and mesostructured skeletons prepared by combining the alkoxy-derived sol-gel process accompanied by phase separation and the solvothermal process, Chem. Mater. 20 (2008) 2165-2173. |




