The synthesis and development of new energetic materials with higher performance and lower sensitivity toward thermal shock and friction has gained considerable recent interest. However,the parameters of insensitivity and high energy content are often mutually exclusive,which makes the development of new highenergy materials a challenging problem. As a consequence, attention has been devoted to nitrogen-rich heterocycles,owing to their low vapor pressures,higher enthalpies of formation,and enhanced thermal stabilities [1–3]. The decomposition of nitrogenrich compounds generates large volumes of environmentally friendly N2,which makes them promising candidates for applications that require environmental friendly and ‘‘green’’ energetic materials.
As a known compound,2-azidoimidazole is commonly referred to AIM and is useful as an intermediate in the production of explosives [4, 5, 6]. In 2002,a salt formed by AIM and perchloric acid was reported by Hammerl and Klapö tke [4]. In 2012,Tang and coworkers [5, 6] obtained three new energetic compounds (AIM)NO3, (AIM)(HTNR) and (AIM)(PA) by reacting 2-azido-imidazolium with HNO3,2,4,6-trinitroresorcinol (TNR) and picric acid (PA).
With the intent of using the advantageous properties of azidoimidazole and to study their structure–property relationships, we have synthesized 2-azido-4-nitroimidazole (ANI),and reported the synthesis,characterization,and energetic properties of nitrogen-rich energetic salts of 2-azido-4-nitroimidazole (ANI) (Scheme 1).

|
Download:
|
| Scheme 1.Synthesis of energetic salts of ANI. | |
Caution! The titled compounds are energetic material and exhibit a tendency to explode under certain conditions. Proper protective measures (safety shield,face shield,leather gloves,and protective clothing,such as heavy leather welding suits and ear plugs) should be taken during the synthesis,testing and measurement processes,especially when these compounds are prepared on a larger scale. 2.1. Materials and instruments
The starting materials used in the present study were of AR grade and purchased from the trade. Melting points were measured on an X-4 melting point apparatus and were uncorrected. IR spectra were recorded on IR Prestige-21 instrument. 1H NMR (500 MHz) and 13C NMR (125 MHz) were recorded on Bruker Avance Spectrometer (TMS as an internal standard). Elemental analysis was carried out on Perkin-Elmer instrument. 2.2. Synthesis
Preparation of 2-azidoimidazole: Water (10 mL) was placed in a round-bottomed flask. With stirring,1 mL of concentrated sulfuric acid was added,followed by 0.81 g (4.5 mmol) 2-aminoimidazole sulfate and the suspension was cooled to 0–5 °C in an ice-salt bath.
A solution of 0.33 g (4.8 mmol) of sodium nitrite in 3 mL of water was added dropwise,and the mixture was stirred for an additional 45 min. With strong stirring,a solution of 0.36 g (5.1 mmol) of sodium azide in 3 mL of water was added,and stirring was continued for an additional 40 min. The reaction mixture was poured into an ice–water mixture,then extracted 3 times with acetic ether. The extracts were dried over anhydrous magnesium sulfate and evaporated to give an isolated product of 0.44 g. Yield: 89.8%; mp84–86°C; 1H NMR (500 MHz,acetone-d6): ° 6.90 (s,1H), 10.97 (s,1H).
Preparation of 2-azido-4-nitroimidazole (ANI): Sulfuric acid (98%,3 mL) was added dropwise to a well stirred mixture of 2- azidoimidazole (0.2 g,1.3 mmol) and nitric acid (68%,2 mL) at room temperature for 4 h. The reaction mixture was poured into an ice–water mixture,then extracted 3 times with acetic ether. The extracts were dried over anhydrous magnesium sulfate and evaporated to give a product of 0.24 g. Yield: 85.7%; mp 141– 143 8C; 1H NMR (500 MHz,DMSO-d6): &delat; 8.27 (s,1H),13.46 (s,1H); 13C NMR (125 MHz,DMSO-d6): &delat; 118.63,140.06,144.50; anal. calcd. for C3H2N6O2: C,23.38; H,1.31; N,54.54; found: C,23.26; H, 1.39; N,54.56%.
Preparation of ammonium ANI (1): A solution of ANI (0.16 g, 1 mmol) in MeOH (10 mL) was slowly added to a mixture of ammonium carbonate (1 mmol) in MeOH (5 mL) at 25°C with stirring. After stirring for 4 h at 50°C,the solvent was removed in vacuo and light yellow plate crystals were obtained after recrystallization from water (0.15 g). Yield: 83.3%; mp 152– 154°C; IR (KBr,cm-1): 3147,2135,1564,1522,1497,1455, 1440,1369,1354,1232,1204,1106,1017,1001,789,753,701, 572,544; 1H NMR (500 MHz,D2O): &delat; 7.89 (s,1H); 13C NMR (125 MHz,D2O): &delat; 117.96,141.12,143.65; anal. calcd. for C3H5N7O2: C,21.06; H,2.95; N,57.30; found: C,21.09; H,2.99; N,57.26.
Preparation of guanidinium ANI (2): A solution of ANI (0.16 g, 1 mmol) in MeOH (10 mL) was slowly added to a mixture of the guanidine carbonate (0.09 g,1 mmol) in MeOH (5 mL) at 25°C with stirring. After stirring for 4 h at 65°C,the solvent was removed in vacuo and light yellow plate crystals were obtained after recrystallization from water (0.18 g). Yield: 81.8%; mp 135– 137°C; IR (KBr,cm-1): 3484,3338,2974,2134,1645,1493,1460, 1401,1367,1314,1204,1145,854,778,755,714,630,599; 1H NMR (500 MHz,D2O): &delat; 7.83 (s,1H); 13C NMR (125 MHz,D2O): &delat; 133.50,144.34,149.93,157.01; anal. calcd. for C4H7N9O2: C,22.54; H,3.31; N,59.14; found: C,22.56; H,3.35; N,59.11. Preparation of nitrate ANI (3): A solution of ANI (0.16 g, 1 mmol) in MeOH (10 mL) was slowly added to a solution of the nitric acid (0.07 mL,1 mmol) in MeOH (5 mL) at 25°C with stirring. After stirring for 4 h,the solvent was removed in vacuo and light yellow plate crystals were obtained after recrystallization from water (0.15 g). Yield: 75.5%; mp 122–124°C; IR (KBr,cm-1): 3147, 2136,1564,1523,1497,1440,1394,1353,1232,1204,1106,1001, 789,752,701,572,542; 1H NMR (500 MHz,D2O,): &delat;7.88 (s,1H); 13C NMR (125 MHz,D2O): &delat; 117.83,141.04,143.59; anal. calcd. for C3H3N7O5: C,16.60; H,1.39; N,45.16; found: C,16.63; H,1.42; N,45.11. 3. Results and discussion 3.1. Theoretical study
Calculations were carried out using the Gaussian 09 program suite [7]. The geometric optimization of the structures and frequency analyses were carried out using the B3LYP functional with the 6-31G** basis set [8]. All of the optimized structures were characterized to be true local energy minima on the potentialenergy surface without imaginary frequencies.
Significant progress has been made in the theoretical prediction of the HOFs of energetic salts with good accuracy,together with the HOFs of the cations and anions and the lattice energies of the salts. The lattice potential energies and lattice energies were predicted by using the approach of Jenkins et al. [9]. Based on a Born–Fajans– Haber energy cycle,the heat of formation of a salt can be simplified by using Eq. (1),where ΔHL is the lattice enthalpy of the ionic salt.
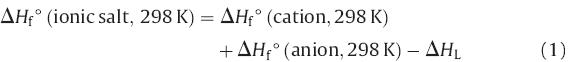


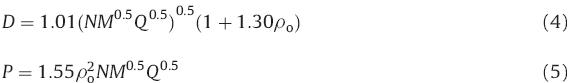
The enthalpy of formation (HOF) of an energetic material provides information regarding its energy content and is important in assessing its detonation performance. To obtain energetic salts with high energy,the anions should also possess high positive HOFs. The gas-phase HOFs were calculated by using a computational approach through isodesmic reactions (Scheme 2). The HOFs for ANI and compounds 1–3 were calculated based on the Born– Fajans–Haber enthalpy cycles (Fig. 1) and are summarized in Table 1. The calculated HOF of ANI (421.75 kJ/mol) was positive, which can be attributed to the high energy contribution from the azido groups. The HOFs exhibited by these energetic salts were within the range 292–338 kJ/mol. The HOFs of 1 and 2 using ANI as an anion were found to be higher than 3 using ANI as a cation. Density is one of the most important factors in determining the performance of energetic compounds. The densities of salts 1–3 were calculated by using the Hofmann approach [10] and were within the range 1.56–1.78 g/cm3 (Table 1). From the calculated HOFs and densities of these new energetic salts,their detonation velocities (D) and detonation pressures (P) were calculated by using the Kamlet–Jacobs equation [11]. The detonation velocities were within the range 7.3–8.6 km/s and their detonation pressures were between 18 and 33 GPa (Table 1). Salt 3 (D = 8.58 km/s, P = 32.45 GPa) exhibited better detonation performance than TNT (D = 7.21 km/s,P = 22.49 GPa) and close to RDX (D = 8.75 km/s, P = 34.7 GPa). Overall,these salts possessed high nitrogen content, high positive HOFs,and moderate performance.

|
Download:
|
| Scheme 2.Representative isodesmic reactions. | |

|
Download:
|
| Fig. 1.Born–Fajans–Haber cycle for the formation of energetic salts (ΔHL = lattice enthalpy for ionic salts. ΔHf° (cation) and ΔHf° (anion) = enthalpy of formation of the cation and anion,respectively). | |
| Table 1 Properties of energetic salts of the ANI. |
In summary,a simple and straightforward approach for the synthesis of 2-azido-4-nitroimidazole (ANI) has been devised. Its salts,which can be easily synthesized and safely handled, exhibited promising physical properties,such as high nitrogen content,and high positive heats of formation. The densities of these salts were within the range 1.56–1.78 g/cm3. The detonation velocities and detonation pressures of salts 1 and 3 were higher than that of TNT. All of the energetic salts examined herein showed moderate performance and high nitrogen content and, hence,may have potential applications in gas generators and pyrotechnics.
| [1] | (a) R.P. Singh, R.D. Verma, D.T. Meshri, J.M. Shreeve, Energetic nitrogen-rich salts and ionic liquids, Angew. Chem. Int. Ed. 45 (2006) 3584-3601; (b) H. Gao, C. Ye, O.D. Gupta, et al., 2,4,5-Trinitroimidazole-based energetic salts, Chem. Eur. J. 13 (2007) 3853-3860; (c) R. Duddu, P.R. Dave, R. Damavarapu, N. Gelber, D. Parrish, Synthesis of Namino-and N-nitramino-nitroimidazoles, Tetrahedron Lett. 51 (2010) 399-401; (d) R. Duddu, M.X. Zhang, R. Damavarapu, N. Gelber, Molten-state nitration of substituted imidazoles: new synthetic approaches to the novel melt-cast energetic material, 1-methyl-2,4,5-trinitroimidazole, Synthesis 17 (2011) 2864-2895. |
| [2] | D. Srinivas, V.D. Ghule, K. Muralidharan, H.D.B. Jenkins, Tetraanionic nitrogen-rich tetrazole-based energetic salts, Chem. Asian J. 8 (2013) 1023-1028. |
| [3] | J.H. Zhang, C.L. He, D.A. Parrish, J.M. Shreeve, Nitramines with varying sensitivities: functionalized dipyrazolyl-N-nitromethanamines as energetic materials, Chem. Eur. J. 19 (2013) 8929-8936. |
| [4] | A. Hammerl, T.M. Klapötke, Tetrazolylpentazoles: nitrogen-rich compounds, Inorg. Chem. 41 (2002) 906-912. |
| [5] | Z. Tang, L. Yang, X.J. Qiao, et al., Crystal structure, thermal decomposition and sensitivity properties of (AIM)(HTNR) and (AIM)(PA), Chem. Res. Chin. Univ. 28 (2012) 4-8. |
| [6] | Z. Tang, L. Yang, X.J. Qiao, et al., Crystal structure and thermal analysis of two new energetic compounds (AIM)NO3 and (AIM)(HTNR) H2O, Acta Chim. Sin. 70 (2012) 471-478. |
| [7] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Gaussian, Inc., Wallingford, CT, 2009. |
| [8] | W.J. Hehre, R. Ditchfield, J.A. Pople, Self-consistent molecular orbital methods. XⅡ. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules, J. Chem. Phys. 56 (1972) 2257-2261. |
| [9] | (a) H.D.B. Jenkins, Thermodynamics of the relationship between lattice energy and lattice enthalpy, J. Chem. Educ. 82 (2005) 950-952; (b) H.D.B. Jenkins, D. Tudela, L. Glasser, Lattice potential energy estimation for complex ionic salts from density measurements, Inorg. Chem. 41 (2002) 2364-2367; (c) L. Glasser, H.D.B. Jenkins, Lattice energies and unit cell volumes of complex ionic solids, J. Am. Chem. Soc. 122 (2000) 632-638; (d) H.D.B. Jenkins, H.K. Roobottom, J. Passmore, L. Glasser, Relationships among ionic lattice energies, molecular (formula unit) volumes, and thermochemical radii, Inorg. Chem. 38 (1999) 3609-3620. |
| [10] | D.W.M. Hofmann, Fast estimation of crystal densities, Acta Crystallogr. B 58 (2002) 489-493. |
| [11] | (a) M.J. Kamlet, S.J. Jacobs, Chemistry of detonations. I. A simple method for calculating detonation properties of C-H-N-O explosives, J. Chem. Phys. 48 (1968) 23-25; (b) M.J. Kamlet, J.E. Ablard, Chemistry of detonations. Ⅱ. Buffered equilibria, J. Chem. Phys. 48 (1968) 36-42. |





