b Nano and Biotechnology Research Group, University of Mazandaran, Babolsar 47416-95447, Iran;
c Department of Microbiology, Faculty of Sciences, University of Mazandaran, Babolsar 47416-95447, Iran
Pyranoquinoline derivatives are known to be present in many natural alkaloids [1, 2, 3, 4],and possess various pharmacological and biological activities such as antimalarial [5],antiseptic [6], antihypertensive [7],antiviral effect and act as H1-antihistamines [8, 9]. Several quinolone derivatives have been reported as antimicrobial agents in the treatment of many infections [10, 11]. They are widely used as synthetic precursors for the construction of many natural polycyclic molecules [12]. Thus, several methods have been described for the synthesis of pyranoquinoline derivatives [13, 14, 15, 16]. Among available methods, intramolecular cyclization via multicomponent reaction is an efficient protocol for constructing these heterocyclic compounds [17, 18, 19, 20, 21]. Moreover,employing appropriate routes to enhance the efficiency of the reactions is a subject of interest. Besides various other reaction parameters,the nature of the catalyst and solvent has significant impact on the reaction [22, 23, 24, 25].
Thus,in continuation to our studies in the synthesis of new heterocycles [26, 27, 28, 29, 30],we report an efficient synthesis of pyranoquinoline derivatives 4 which are obtained through threecomponent reactions of 4-hydroxy-1-methyl-2(1H)-quinolinone 1,aromatic aldehydes 2 and ethyl cyanoacetate 3 catalyzed by 4-dimethyl aminopyridine (DMAP) in H2O/EtOH (1:1) (Scheme 1). Also,some of the selected products were evaluated for their antibacterial activity against Gram positive and Gram negative bacteria.

|
Download:
|
| Scheme 1.Synthesis of pyranoquinoline derivatives 4a–i. | |
Elemental analyses were performed using a Heraeus CHN-ORapid analyzer. NMR spectra were recorded on a BRUCKER DRX- 400 AVANCE spectrometer (at 400.1 for 1H and 100.6 MHz for 13C) with CDCl3 as solvent. Mass spectra were recorded on a Finnigan- Matt 8430 mass spectrometer operating at an ionization potential of 70 eV. IR spectra were recorded on a FT-IR,Brucker,VECTOR 22 spectrometer. 2.1. General procedure for the synthesis of compound 4 (exemplified by 4a)
A mixture of 4-nitrobenzaldehyde (1 mmol) and ethyl cyanoacetate (1 mmol) in H2O/EtOH (1:1) (5 mL) was treated with DMAP (10 mol%) at room temperature. After the consumption of starting aldehyde as indicated by TLC analysis (30 min),4-hydroxy-1- methyl-2(1H)-quinolinone (1 mmol) was added to the reaction mixture and the mixture was heated to reflux condition for 2 h. After the completion of the reaction,the reaction mixture was brought to room temperature and the solid precipitate was collected by filtration. The desired product 4a was obtained as a yellow powder. 2.2. General procedure for evaluation of antibacterial activity
The in vitro biocidal screening,antibacterial activity of compounds 4a,4c,4d,4f and 4g was assayed using the Kirby– Bauer disc diffusion method where a filter disc was impregnated with the compounds and placed on the surface of inoculated agar plates. The synthesized compounds were dissolved in DMSO to make a 20 mg mL-1 solution then filtered through a sterilized 0.22 mm Ministart (Sartorius) filter. The antibacterial activity of the products was investigated against four bacterial species. Test organisms included Escherichia coli PTCC 1330,Pseudomonas aeruginosa PTCC 1074,Staphylococcus aureus ATCC 35923 and Bacillus subtilis PTCC 1023. Late exponential phase of the bacteria was prepared by inoculating 1% (v/v) of the cultures into a fresh Muller–Hinton broth (Merck) and incubating on an orbital shaker at 37°C and 100 rpm overnight. Before using the cultures,they were standardized with a final cell density of approximately 108 cfu mL-1. Muller–Hinton agar (Merck) was prepared and inoculated from the standardized cultures of the test organisms then spread as uniformly as possible throughout the entire media. Sterile paper discs (6 mm diameter,Padtan,Iran) were impregnated with 20 mL of the compound solution then allowed to dry. The impregnated disc was introduced on the upper layer of the seeded agar plate and incubated at 37°C for 24 h. The antibacterial activity of the products was compared with known antibiotic gentamicin (10 mg/disc) and chloramphenicol (30 mg/disc) as positive controls and DMSO (20 mL/disc) as negative control. Antibacterial activity was evaluated by measuring the diameter of the inhibition zone (mm) on the surface of the plates and the results were reported as mean ± SD after three repeats. 3. Results and discussion 3.1. Chemistry
Initially,we investigated different conditions including catalysts and solvents to optimize the reaction. As a model reaction,a mixture of 4-nitrobenzaldehyde,ethyl cyanoacetate and 4- hydroxy-1-methyl-2(1H)-quinolinone was treated in the absence of any catalyst in which trace amount of the product 4a was obtained. Then,the model reaction was performed in the presence of various amounts of DMAP and hexamethylenetetramine (HMT). It was noted that 10 mol% of DMAP provided the best result in terms of yield and time. Using HMT instead of DMAP,the reaction proceeded with a decreased yield (Table 1).
| Table 1 The effects of different catalytic amounts of DMAP and HMT on the reaction of 4- nitrobenzaldehyde,ethyl cyanoacetate and 4-hydroxy-1-methyl-2(1H)-quinolinone in H2O/EtOH (1:1) under reflux. |
The model reaction was optimized using various solvents to obtain the best yield of 4a. Among different solvents,the best results were obtained using H2O/EtOH (1:1) and DMF. Thus,we preferred to carry out the reactions using H2O/EtOH (1:1) as an ecofriendly and safe medium. The results are summarized in Table 2.
| Table 2 The effects of different solvents on the reaction of 4-nitrobenzaldehyde,ethyl cyanoacetate and 4-hydroxy-1-methyl-2(1H)-quinolinone using DMAP (10 mol%) under reflux condition. |
With the optimal reaction conditions,the reactions of some other aromatic aldehydes were carried out with ethyl cyanoacetate and 4-hydroxy-1-methyl-2(1H)-quinolinone and resulted in products 4b–i in excellent yields (Table 3).
| Table 3 Synthesis of dihydropyrano[3,2-c]quinoline derivatives catalyzed by DMAP (10 mol%) in H2O/EtOH (1:1) under reflux condition. |
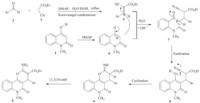
A plausible rationalization for the reaction mechanism is shown in Scheme 2. Presumably,compound 5 is formed via Knoevenagel condensation between aldehyde 2 and ethyl cyanoacetate 3 in the presence of a catalytic amount of DMAP. Then,the enolate 6,which is obtained from the reaction between quinolinone 1 and DMAP, performs the Michael addition to intermediate 5 to generate the intermediate 7. This intermediate enolizes to produce 8,which subsequently undergoes an intramolecular cyclization reaction to form 9. Then,intermediate 9 undergoes a 1,3-proton shift to afford the product 4 exclusively.
|
Download:
|
| Scheme 2.Proposed mechanism for the formation of pyranoquinoline derivatives 4a–i. | |
The structures of compounds 4a–i were consistent with their 1H NMR,13C NMR,IR and mass spectra and elemental analysis. Spectral data of compounds 4a–i are listed in Supporting information.
The 1H NMR spectrum of 4a exhibited a triplet for CH3 at δ 1.11, a singlet for NCH3 at δ 3.55,a multiplet at δ 3.97–4.02 for OCH2,a singlet for CH at δ 4.96,a multiplet for aromatic protons at δ 7.40– 8.13 and a singlet for NH2 protons at δ 7.90. The 13C NMR spectrum of 4a displayed 20 resonances in agreement with the proposed structure. Infrared spectral data of 4a displayed four absorption bands at 3374,3276,1696 and 1624 cm-1 indicating the presence of NH2 and carbonyl groups,respectively. The mass spectrum displayed a molecular ion peak at m/z 421. The 1H NMR and 13CNMR spectra of 4b–i are similar to those of 4a,except for the signals related to the substituents of the aldehydes. 3.2. Antibacterial activity
The in vitro antibacterial activity of compounds 4a,4c,4d,4f and 4g was evaluated against Gram positive S. aureus and B. subtilis and Gram negative E. coli and P. aeruginosa. The inhibition of microorganism growth was benchmarked against the standard antibiotics gentamicin and chloramphenicol (Table 4) [31, 32]. The results revealed that the synthesized compounds have moderate to good bacteriostatic effects against some microorganisms,and among the bacteria tested,the compounds exhibited the highest antibacterial activity against P. aeruginosa. The activity of compound 4g against all test bacteria was good whereas the compound 4a shows low activity against P. aeruginosa and no effect against S. aureus (Table 4).
| Table 4 Antibacterial activities of the selected products using Kirby–Bauer disc diffusion method. |
In conclusion,we have described a convenient method for the synthesis of dihydropyrano[3,2-c]quinoline derivatives by applying a three-component reaction of 4-hydroxy-1-methyl-2(1H)- quinolinone,aromatic aldehydes and ethyl cyano acetate in the presence of a catalytic amount of DMAP in aqueous ethanol. The simplicity of the reaction,easy separation procedure,excellent yields of the products,and short reaction time make it an efficient rout for synthesizing pyranoquinoline heterocycles. The observed antibacterial properties suggest that the methoxy derivative of the synthesized pyranoquinolines exhibited good antibacterial activity and can be further developed as an effective antimicrobial agent. Acknowledgment
This research was supported by the Research Council of the University of Mazandaran in Iran.
| [1] | A. McKillop, L. McLaren, R.J. Watson, R.J. Taylor, N. Lewis, A concise synthesis of the novel antibiotic aranorosin, Tetrahedron Lett. 34 (1993) 5519-5522. |
| [2] | I.S. Chen, S.J. Wu, I.J. Tsai, et al., Chemical and bioactive constituents from Zanthoxylum simulans, J. Nat. Prod. 57 (1994) 1206-1211. |
| [3] | M.F. Grundon, The Alkaloids: Quinoline Alkaloids Related to Anthranilic Acid, Academic Press, London, 1988, pp. 341-439. |
| [4] | W.N. Setzer, B. Vogler, R.B. Bates, et al., HPLC-NMR/HPLC-MS analysis of the bark extract of Stauranthus perforates, Phytochem. Anal. 14 (2003) 54-59. |
| [5] | A. Afonso, S.W. McCombie, J. Weinstein, Quinoline-diones, US Patent 5179093A (1993). |
| [6] | G.C. Sharp, Antifungal methods employing certain carbostyrils, US Patent 3836657A (1974). |
| [7] | C. Jolivet, C. Rivalle, E. Bisagni, Synthesis of pyrano[2,3-h]quinolines as tricyclic acronycine analogues, Heterocycles 43 (1996) 995-1005. |
| [8] | A. Afonso, J. Weinstein, M.J. Gentles, Alkyl and acyl substituted quinolines, US Patent 5382572A (1995). |
| [9] | M. Miyoshi, N. Yoneda, R. Matsumoto, M. Suzuki, 3-Amino-4-hydroxycarbostyril derivatives, Tanabe Seiyaku Co. Ltd., Jpn. Kokai, 7746083 (1977). |
| [10] | S.D. Mathada, B.H. Mathada, Synthesis and antimicrobial activity of some 5-substituted-3-phenyl-Nb-(substituted-2-oxo-2H-pyrano[2,3-b]quinoline-3-carbonyl)-1H-indole-2-carboxyhydrazide, Chem. Pharm. Bull. 57 (2009) 557-560. |
| [11] | K.L. Hopkins, R.H. Davies, E.J. Threfall, Mechanisms of quinolone resistance in Escherichia coli and Salmonella: recent developments, Int. J. Antimicrob. Agents 25 (2005) 358-373. |
| [12] | M. Ramesh, P.S. Mohan, P. Shanmugam, A convenient synthesis of flindersine, atanine and their analogues, Tetrahedron 40 (1984) 4041-4049. |
| [13] | X.F. Duan, J. Zeng, Z.B. Zhang, G.F. Zi, A facile two-step synthesis of 2-arylbenzofurans based on the selective cross McMurry couplings, J. Org. Chem. 72 (2007) 10283-10286. |
| [14] | P. Roy, B.K. Ghorai, One-pot synthesis of pyrano[4,3-b]quinolinones from 2-alkynyl-3-formylquinolines via oxidative 6-endo-dig ring closure, Tetrahedron Lett. 53 (2012) 235-238. |
| [15] | J.H. Ye, K.Q. Ling, Y. Zhang, N. Li, J.H. Xu, Syntheses of 2-hydroxypyrano[3,2-c]quinolin-5-ones from 4-hydroxyquinolin-2-ones by tandem Knoevenagel condensation with aldehyde and Michael addition of enamine with the quinone methide-thermo-and photochemical approaches, J. Chem. Soc., Perkin Trans. 1 (1999) 2017-2024. |
| [16] | X.S. Wang, Q. Li, J.R. Wu, S.J. Tu, Efficient method for the synthesis of pyranoquinoline, thiopyranoquinoline, thienoquinoline, and naphtho[2,7]naphthyridine derivatives catalyzed by iodine, J. Comb. Chem. 11 (2009) 433-437. |
| [17] | L. El Kaim, L. Grimaud, X.F.L. Goff, A. Schiltz, Smiles cascades toward heterocyclic scaffolds, Org. Lett. 13 (2011) 534-536. |
| [18] | I.V. Ukrainets, R.G. Red'kin, L.V. Sidorenko, A.V. Turov, 4-Hydroxy-2-quinolones 172. Synthesis and structure of 4,3'-spiro[(6-allyl-2-amino-5-oxo-5,6-dihydro-4h-pyrano-[3,2-c]quinoline-3-carbo-nitrile)-20-oxindole], Chem. Heterocycl. Compd. 45 (2009) 1478-1484. |
| [19] | A.T. Khan, M. Lal, S. Ali, M.M. Khan, One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst, Tetrahedron Lett. 52 (2011) 5327-5332. |
| [20] | E. Altieri, M. Cordaro, G. Grassi, F. Risitano, A. Scala, Regio and diastereoselective synthesis of functionalized 2,3-dihydrofuro[3,2-c]coumarins via a one-pot threecomponent reaction, Tetrahedron 66 (2010) 9493-9496. |
| [21] | I.V. Magedov, M. Manpadi, M.A. Ogasawara, et al., Structural simplification of bioactive natural products with multicomponent synthesis. 2. Antiproliferative and antitubulin activities of pyrano[3,2-c]pyridones and pyrano[3,2-c]quinolones, J. Med. Chem. 51 (2008) 2561-2570. |
| [22] | L. El Kaïm, L. Grimaud, S. Wagschal, Toward pyrrolo[2,3-d]pyrimidine scaffolds, J. Org. Chem. 75 (2010) 5343-5346. |
| [23] | T. Godet, C. Vaxelaire, C. Michel, A. Milet, P. Belmont, Silver versus gold catalysis in tandem reactions of carbonyl functions onto alkynes: a versatile access to furoquinoline and pyranoquinoline cores, Chem. Eur. J. 13 (2007) 5632-5641. |
| [24] | S. Banerjee, A. Horn, H. Khatri, G. Sereda, A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst, Tetrahedron Lett. 52 (2011) 1878-1881. |
| [25] | M. Yoshida, Y. Fujino, K. Saito, T. Doi, Regioselective synthesis of flavone derivatives via DMAP-catalyzed cyclization of o-alkynoylphenols, Tetrahedron 67 (2011) 9993-9997. |
| [26] | S. Asghari, M. Qandalee, Three-component, one-pot synthesis of new functionalized pyrrolines, Synth. Commun. 40 (2010) 2172-2177. |
| [27] | S. Asghari, A.K. Habibi, Synthesis of halogenated α,β-unsaturated γ-butyrolactone derivatives by triphenylphosphine-catalyzed cyclization of α-halogeno ketones with dialkyl acetylenedicarboxylates, Helv. Chim. Acta 95 (2012) 810-817. |
| [28] | S. Asghari, A. Khabbazi Habibi, One pot three-component regioselective and diastereoselective synthesis of halogenated pyrido[2,1-b][1,3]oxazines, Tetrahedron 68 (2012) 8890-8898. |
| [29] | S. Asghari, M. Qandalee, Z. Naderi, Z. Sobhaninia, One-pot synthesis of 4-arylquinolines from aromatic aminoketones and vinylphosphonium salts, Mol. Divers. 14 (2010) 569-574. |
| [30] | S. Asghari, M. Tajbakhsh, V. Taghipour, A facile route to N-acetyl a,b-unsaturated g-lactam derivatives using ethyl acetamidocyanoacetate and dialkyl acetylenedicarboxylate in the presence of triphenylphosphine, Tetrahedron Lett. 49 (2008) 1824-1827. |
| [31] | F.C. Tenover, Antibiotic susceptibility testing, in: Encyclopedia of Microbiology, 3rd ed., Academic Press, Oxford, 2009p. 67. |
| [32] | M. Ghaemy, B. Aghakhani, M. Taghavi, S.M.A. Nasab, M. Mohseni, Synthesis and characterization of new imidazole and fluorine bisphenol based polyamides: thermal, photophysical and antibacterial properties, React. Funct. Polym. 73 (2013) 555-563. |