Cyclic nitramines constitute an important class of highly energetic materials whose synthesis and properties have been extensively studied. 1,4,5,8-Tetranitro-1,4,5,8-tetraazabicyclo- [4,4,0]-decane was first synthesized by Willer [1] and it is an energetic compound used as a main ingredient in composite explosives and propellants. TNAD can yield a lower pressure exponent than that of RDX and HMX,the rate of change in space of the temperature gradient is negligible for these propellants and the rate of mass diffusion is believed to be smaller compared with the rate of mass convection [2, 3]. The compatibility of TNAD with other energetic components and inert materials is one of the most stringent aspects of TNAD [4]. In practical applications,it is responsible for releasing the reactivity in these compounds when detonated [5].
The synthesis of TNAD using conventional reagents involved the nitrolysis of 1,4,5,8-tetranitroso-1,4,5,8-tetraazabicyclo- [4,4,0]-decane [1, 6, 7] and the nitration of 1,4,5,8 -tetraazabicyclo-[ 4,4,0]-decane with HNO3/Ac2O as the nitrating reagents [8]. However,the yields of nitrolysis reactions are below 60% and the sequence involves more than two steps. The yield of the first step of the nitrolysis is very high,but the disposal is difficult because it generates HCl. The yield of the second step is low and all nitrolyses employ HNO3 or H2SO4 as solvent. The yields can be improved in the presence of HNO3–Ac2O,but this reaction requires a large excess of nitric acid and acetic anhydride in more than 2 h,which adds considerable expense. Also the high reaction temperature makes the reaction dangerous. In short,these methods inevitably showed some weaknesses such as poor yield,environmental pollution,low reaction rate,and inconvenient waste disposal.
In recent years,various cleaner nitration approaches have been explored. N2O5 employed as a green nitrating reagent had been studied for several years. Millar et al. [9] introduced N2O5 in an inert solvent into the synthesis of nitramines and nitrate esters. Talawer et al. [10] succeeded in finding several N2O5–inert solvents as green and mild systems. Zhi et al. [11] reported that PEG200- DAIL with N2O5 could increase the yield of HMX significantly. Cheng et al. [12] improved the yield of RDX by 10.5% using ionic liquid as a catalyst.
The past few years have witnessed a growing interest in ionic liquids (ILs) as solvent and catalyst for certain organic reactions. In the presence of ionic liquids,the conversions of the nitration of aromatic compounds have a significant improvement [13–17].
Here we combined the advantage of acidic ionic liquid with N2O5-inert solvents. We attempted nitration of 1,4,5,8-tetrazabicyclo-[ 4,4,0]-decane using the green nitrating agent,dinitration pentoxide,in the presence of acidic ionic liquids. In this reaction, the temperature is lower,there is no acidic waste and the solvent can be used repeatedly. The results are presented in this letter. 2. Experimental
All chemicals were purchased from commercial sources and used for the reaction without further purification. The melting points were obtained with a WRS-1B Digital Melting Apparatus. The IR spectra were recorded on a Nexus 870FT-IR spectrometer and expressed in cm-1. 1H NMR was recorded on a Bruker DRX 500 MHz spectrometer. 2.1. Preparation of N2O5 [18]
P2O5 was added slowly to a distillation flask containing freshly distilled nitric acid (100%) and reaction temperature should be kept below 10°C. Slow distillation affords N2O5 with the liberation of nitrogen oxides. The collected N2O5 was a white/yellow solid. The product was warmed slowly to 10°C so that any liquid nitrogen oxides were discarded. It is important that the N2O5 used is free of nitrogen oxides. Caution: N2O5 is highly corrosive and liberates toxic nitrogen oxide. Small amounts can be quickly neutralized with copious amount of water. 2.2. Preparation of acidic ionic liquids
[HMim]X (X- = pTSO-,NO3-,HSO4-) were synthesized according to the procedures reported by Lu et al. [12].
[HMim]pTSO: 1H NMR (500 MHz,D2O,TMS): d 2.21 (s,3H),3.69 (s,3H),7.17 (d,2H),7.20 (d,2H),7.54 (s,2H),8.31 (s,1H).
[HMim]NO3: 1H NMR (500 MHz,D2O,TMS): δ 3.80 (s,3H),7.46 (s, 2H),8.70 (s,1H). [HMim]HSO4: 1H NMR (500 MHz,D2O,TMS): d 3.69 (s,3H),7.21 (s,2H),8.43 (s,1H).
[(CH2)4SO3HMim]X (X- = pTSO-,NO3 -,HSO4-) were synthesized according to the procedures reported by Qi et al. [19].
[(CH2)4SO3]HSO4: 1H NMR (500 MHz,D2O,TMS): δ 1.46 (m,2H),1.51 (t,2H),2.11 (s,3H),2.69 (t,2H),3.60 (s,3H),7.08 (d, 1H),7.18 (d,2H),7.42 (d,2H),8.40 (s,1H). [(CH2)4SO3HMim]pTSO: 1H NMR (500 MHz,D2O,TMS): δ 2.03 (s,3H) 2.13 (m,2H),2.76 (t,2H),3.75 (s,3H),4.12 (t,2H) 7.15 (d,2H),7.21 (d,2H) 7.38 (s, 2H),8.34 (s,1H).
[Capl]X (X- = pTSO-,NO3-,HSO4-) were synthesized according to the procedures reported by Cheng et al. [20].
[Capl]pTSO: 1H NMR (500 MHz,D2O,TMS): δ 1.61–1.77 (m,6H), 2.41 (s,3H),2.49 (t,2H),3.26 (t,2H),7.38 (d,2H),7.71 (d,2H). [Capl]NO3: 1H NMR (500 MHz,D2O,TMS): δ 1.41–1.61 (m,6H), 2.34 (t,2H),3.10 (t,2H). [Capl]HSO4: 1H NMR (500 MHz,D2O, TMS): δ 1.37–1.57 (m,6H),2.16 (t,2H),3.02 (t,2H). 2.3. Preparation of 1,4,5,8-tetraazabicyclo-[4,4,0]-decane [8]
This compound was obtained as a white powder,mp 205– 207.6°C [lit. [8]: 210–230°C]. IR (KBr,cm-1): n 3160(s),2940(s), 2890(s),2800(s),1490(m),1340(m),1310(w),1150(s),950(s), 860(s). 2.4. General procedure for the synthesis of TNAD catalyzed by acidic ionic liquids
To a cold (0°C) vigorously stirred solution of N2O5 (30 mmol) and CH2Cl2 (30 mL),an indicated amount of ionic liquid was added. A given amount of 1,4,5,8-tetraazabicyclo-[4,4,0]-decane was added in portions to keep the temperature below 5°C. The reaction was carried out at 25°C for 60 min,and then the mixture was cooled to 20°C and poured into 50 g of ice water. The solid product was collected by filtration and rinsed with water,the pure product was obtained after recrystallization from DMSO and dried in vacuum. Mp 231.5–232.3°C; IR (KBr,cm-1): n 3040(w), 2960(w),1550(s),1280(s),1060(m); 1H NMR (500 MHz,DMSO, TMS): δ 6.5 (s,2H),4.4 (m,4H),2.5 (m,4H). 13C NMR (125 MHz, DMSO,TMS): δ 46.9,69.9. 3. Results and discussion
In our initial study,the nitration of 1,4,5,8-tetraazabicyclo- [4,4,0]-decane (prepared according to [8]) using N2O5 in inert solvent in the range of reaction temperatures,reaction times, molar ratios and solvents were examined.
It can be seen from Fig. 1 that the yield of TNAD increased and the reaction rate decreased gradually with time. Most of the reaction was occurred in 50 min. The yield was 77.6% and did not change after 60 min.

|
Download:
|
| Fig. 1.The effect of reaction time on the nitration. Reaction conditions: n(1,4,5,8- tetraazabicyclo-[4,4,0]-decane)/n(N2O5) = 1:6,N2O5/CH2Cl2 (30 mmol/30 mL), 25°C. | |
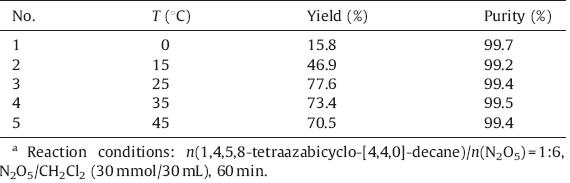
Subsequently the effect of reaction temperature on nitration was explored and the results are shown in Table 1. When the reaction temperature was 0°C,only 15.8% yield was obtained in 60 min and the yield increased as the reaction temperature increased from 0°C to 25°C. Continuing to rise the reaction temperature had an adverse effect on the yield. The reason may be higher temperature made the N2O5 decomposed more rapidly.
| Table 1 Effect of reaction temperature on the nitratio.a |
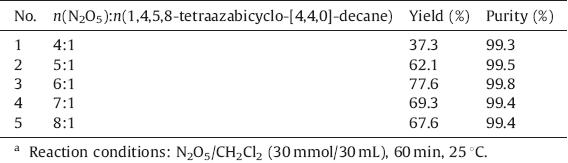
The effect of molar ratio of N2O5 to 1,4,5,8-tetraazabicyclo- [4,4,0]-decane was investigated and the results are summarized in Table 2. It was observed that the yields were improved significantly as the amount of N2O5 increased. The reason was that N2O5 could produce more NO2 +,which was a good nitrating agent that can improve the yield of TNAD. However,excessive N2O5 resulted in lower yield. That may be because of that fact that higher concentration N2O5 cleaved the cyclic structure of the product.
| Table 2 Effect of molar ratio on the nitration.a |
Table 3 shows the results of the nitration of 1,4,5,8-tetrazabicyclo-[ 4,4,0]-decane using N2O5 in various inert solvents.
It was observed that the yields were improved significantly in more polar solvents. The reason was that polar solvent could promote the production of NO2 + from N2O5. The yield of TNAD reached 83.2% in the presence of CH3NO2.
| Table 3 Effect of solvent on the nitration.a |
Numerous papers have reported that strong acidic catalysts could promote the formation of electrophilic nitronium ions (NO2 +) from N2O5 [21, 22]. The effect of the amount of ionic liquid on the nitration of 1,4,5,8-tetrazabicyclo-[4,4,0]-decane was investigated using [(CH2)4SO3]HSO4 in the range of 1%–5% mol ratio to 1,4,5,8-tetrazabicyclo-[4,4,0]-decane,and the results are shown in Fig. 2. It was observed that the yield could be increased to 89.4%,however,further increasing the [(CH2)4SO3HMim] HSO4 loading would decrease the yield. The reason may be that an appropriate acidity was necessary for the nitration reaction. It is important to emphasize that ILs must be used dry to provide the yield achieved.

|
Download:
|
| Fig. 2.Effect of the equivalents of ionic liquid on the nitration. Reaction conditions: n(1,4,5,8-tetraazabicyclo-[4,4,0]-decane)/n(N2O5) = 1:6,60 min,25°C,N2O5/ CH3NO2(30 mmol/30 mL). | |
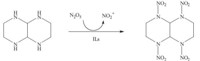
Then the reaction was compared in several IL systems. Based on the data in Table 4,the conclusion could be drawn that all ILs have catalytic activity in the nitration. Compared to the system without ILs,the yield of TNAD increased by 3.0%–6.2% under the mild conditions indicated in Table 4. The IL [(CH2)4SO3HMim]X showed better catalytic activity than other ILs. This might be related to the acidity of the cations (Scheme 1).
| Table 4 Nitration of 1,4,5,8-tetraazabicyclo-[4,4,0]-decane in various ILs.a |
|
Download:
|
| Scheme 1.Possible mechanism for electrophilic nitration. | |
In conclusion,a new efficient process to synthesize TNAD from 1,4,5,8-tetraazabicyclo-[4,4,0]-decane in 89.4% yield was successfully developed using N2O5 and [(CH2)4SO3]HSO4 in CH3NO2. The combination of IL and N2O5 seems to be a useful nitrating system. However,the nitration of other nitrogen heterocyclic compounds using this mild reaction system should be further studied. Acknowledgment
We are grateful for the financial support from the Nature Science Foundation of Jiangsu Province (No. BK2011697).
| [1] | R.L. Willer, Synthesis and characterization of high energy compounds TNAD, Chin. J. Explos. Propell. 8 (1983) 65-69. |
| [2] | Q.L. Yan, X.J. Li, Y. Wang, W.H. Zhang, F.Q. Zhao, Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines: the effect of heat and mass transfer to the burning characteristics, Combust. Flame 156 (2009) 633-641. |
| [3] | L. Qiu, W.H. Zhu, J.J. Xiao, Molecule dynamics simulations of trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin-based polymer-bonded explosives, J. Phys. Chem. B. 111 (2006) 1559-1566. |
| [4] | Q.L. Yan, X.J. Li, L.Y. Zhang, Compatibility study of trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin(TNAD) with some energetic components and inert materials, J. Hazard. Mater. 160 (2008) 529-534. |
| [5] | S. Zeman, Z. Friedl, A new approach to the application of molecular surface electrostatic potential in the study of detonation, Propell. Explos. Pyrot. 37 (2012) 609-613. |
| [6] | Y.Y. Wei, M. Lu, C.X. Lü, Z. Li, Study on synthetic technology of TNAD, in: Theory and Practice of Energetic Materials, Beijing University of Science and Technology, Beijing, 1996. |
| [7] | C. Cai, C.X. Lü, Nitrolysis with nitrogen pentoxide for synthesis of 1,4,5,8-teranitro-1,4,5,8-tetraazabicyclo-[4,4,0]-decalin, Chin. J. Explos. Propell. 28 (2005) 50-51. |
| [8] | M. Lu, C.X. Lü, The improvement of technology for synthesising 1 4,6,9-tetranitro-1,4,6,9-tetraazabicyclo-[4,4,0]-decane, J. Nanjing Univ. Sci. Technol. 21 (1997) 110-113. |
| [9] | R.W. Millar, S.P. Philbin, Novel syntheses of nitramines and nitrate esters by nitrodesilylation reactions using dinitrogen pentoxide(N2O5), Tetrahedron 53 (1997) 4371-4386. |
| [10] | M.B. Talawar, R. Sivabalan, B.G. Polke, et al., Establishment of process technology for the manufacture of dinitrogen pentoxide and its utility for the synthesis of most powerful explosive of today-CL-20, J. Hazard. Mater. 124 (2005) 153-164. |
| [11] | H.Z. Zhi, J. Luo, J.A. Feng, C.X. Lü, An efficient method to synthesize HMX by nitrolysis of DPT with N2O5 and a novel ionic liquid, Chin. Chem. Lett. 20 (2009) 379-382. |
| [12] | G.B. Cheng, X.F. Qi, C.X. Lü, Synthesis of RDX catalyzed by Bronsted acidic ionic liquids, J. Energy Mater. 28 (2010) 35-44. |
| [13] | N.L. Lancaster, V. Llopis-Mester, Aromatic nitrations in ionic liquids: the importance of cation choice, Chem. Commun. 22 (2003) 2812-2813. |
| [14] | M.J. Earle, S.P. Katdare, K.R. Seddon, Paradigm confirmed: the first use of ionic liquids to dramatically influence the outcome of chemical reactions, Org. Lett. 6 (2004) 707-710. |
| [15] | K. Smith, S.F. Liu, G.A. EL-Hiti, Regioselective mononitration of simple aromatic compounds under mild conditions in ionic liquids, Ind. Eng. Chem. Res. 44 (2005) 8611-8615. |
| [16] | K.K. Laali, J.V. Gettwert, Electrophilic nitration of aromatics in ionic liquid solvents, J. Org. Chem. 66 (2001) 35-40. |
| [17] | K. Qiao, C. Yokoyama, Nitration of aromatic compounds with nitric acid catalyzed by ionic liquids, Chem. Lett. 33 (2004) 808-810. |
| [18] | R.R. Bak, A.J. Smallridge, A fast and mild method for the nitration of aromatic rings, Tetrahedron Lett. 42 (2001) 6767-6769. |
| [19] | X.F. Qi, G.B. Cheng, C.X. Lü, Nitration of toluene with nitric acid in the presence of acidic ionic liquid, Chin. J. Energy Mater. 16 (2008) 398-400. |
| [20] | G.B. Cheng, D.S. Qian, C.X. Lü, Nitration of simple aromatics with NO2 under air atmosphere in the presence of novel Bronsted acidic ionic liquids, Synth. Comm. 38 (2008) 537-545. |
| [21] | M.G. Kuba, R. Prins, G.D. Pirngruber, Gas-phase nitration of toluene with zeolite beta, Appl. Catal. A 333 (2007) 78-89. |
| [22] | B. Barletta, E. Bolzacchini, S. Meinardi, M. Orlandi, B. Rindone, The NO3 radicalmediated liquid phase nitration of phenols with nitrogen dioxide, Environ. Sci. Technol. 34 (2000) 2224-2230. |