b Department of Pharmacy Engineering, Shenyang Pharmaceutical University, Shenyang 110016, China
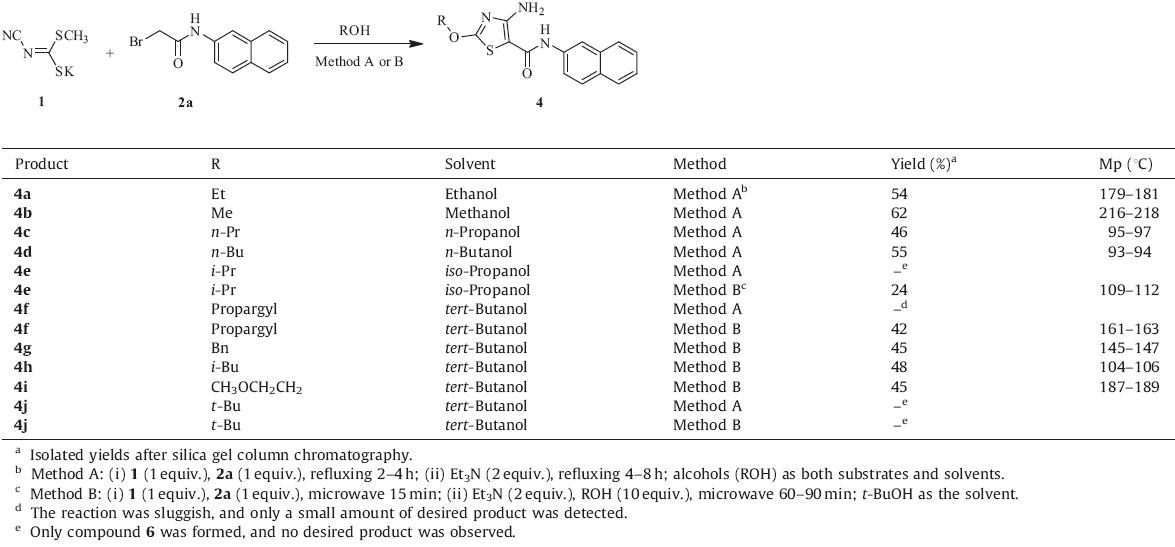
The thiazole ring system as a privileged structure was often explored in the drug discovery process [1,2,3,4,5],and a number of the marketed drugs contained a thiazole fragment [2,6,7]. So far,a great deal of diversified thiazole derivatives were produced and demonstrated a variety of biological activities such as cytotoxic, immunosuppressive and antifungal activities [1,8,9,10]. Pin1 (Protein interaction with NIMA1) is a peptidyl-prolyl cis/trans isomerase and inhibition of Pin1 is a potential therapeutic strategy for the treatment of cancer [11,12,13,14,15]. In our efforts to search for novel thiazoles as Pin1 inhibitors,the desired intermediate 3 was not obtained according to Scheme 1,while unexpectedly,4-amino- 2-ethoxy-N-(naphthalene-2-yl)thiazole-5-carboxamide (4a) was isolated in 54% yield. To our best knowledge,there were few 2- alkoxy-4-amino-N-arylthiazole-5-carboxamides reported in the literature [16,17]. Herein,we wish to present our detailed investigations on the synthesis of the title thiazole derivatives by a simple and efficient protocol.
|
Download:
|
| Scheme 1.Reagents and conditions: (i) ethanol,reflux,1.5 h; (ii) Et3N/ethanol,reflux,4 h. | |
In this work,two different methods were developed to access the thiazole derivatives 4a–i and 4k–r as shown in Tables 1 and 2. The general procedure of method A was given as follows: A mixture of potassium methyl cyanimidodithiocarbonate 1 (1 equiv.) and 2- halo-N-arylacetamides 2 [18–20] (1 equiv.) was heated for 2–4 h using various alcohols (ROH) as reaction solvents. After that,2 equivalents of triethylamine were added and the resulting reaction mixture was refluxed for further 4–8 h to furnish the desired molecules (4a–d and 4k–r) in 41–84% yields. Method B: A mixture of compound 1 (1 equiv.) and 2-bromo-N-(naphthalen-2-yl)- acetamide 2a (1 equiv.) in 2 mL of tert-butanol was loaded into the sealed reaction tube and irradiated by microwave for 15 min (power 60 W,temperature 120°C,pressure 150 psi). And then,10 equivalents of various alcohols as reactants and 2 equivalents of triethylamine were added. The resulting reaction mixture was irradiated by microwave at the same reaction conditions for 60– 90 min to afford the corresponding final products (4e–i) in 24–48% yields.
| Table 1 Synthesis of 2-alkoxy-4-amino-N-(naphthalene-2-yl)thiazole-5-carboxamide derivatives. |
| Table 2 Synthesis of 4-amino-2-ethoxy-N-arylthiazole-5-carboxamide derivatives via method A.a |
With an aim to explore the scope of this protocol,the substituent variations on the 2-position and 5-position of thiazoles were conducted by using a series of alcohols (Table 1,compounds 4a–j) and aryl amines (Table 2,compounds 4k–r),respectively. The chemical structures,synthetic methods and yields of all the obtained 2-alkoxy-4-amino-N-arylthiazole-5-carboxamides were summarized in Tables 1 and 2 [21]. As shown in Table 1,when the unbranched primary alcohols such as methanol,ethanol,n-propanol and n-butanol were tested by functioning as both substrates and solvents in method A,the desired 2-alkoxy substituted thiazoles were obtained in moderate yields (46–62%). However,when the branched iso-propanol or tertbutanol were selected as a substrate,the desired compounds 4e or 4j was not found; on the contrary,N-(3-(naphthalen-2-yl)-4- oxothiazolidin-2-ylidene)cyanamide 6 as shown in Scheme 2 was isolated,probably due to the steric hindrance of iso-propanol and tert-butanol. To our delight,compound 4e was obtained in 24% yield under microwave irradiation,although compound 4j remained elusive in the reaction. Moreover,the initial attempt to introduce propargyloxy substituent onto the 2-position of thiazole scaffold via method A resulted in a sluggish reaction; but the desired product was generated in 42% yield when the reaction was promoted by microwave. In order to further diversify the alkoxy groups on the 2-poistion of thiazoles with different alcohols,method B was developed. In this method,10 equivalents of various alcohols were used as substrates together with starting materials 1 and 2a,and the reactions were carried out in tertbutanol as the reaction medium under microwave irradiation.

|
Download:
|
| Scheme 2.A plausible mechanism proposed for the formation of 4-amino-2-ethoxy-N-(naphthalene-2-yl)thiazole-5-carboxamide (4a). | |
According to this strategy,compounds 4f–i bearing structural diversities were produced in moderate yields (42–48%). Subsequently,a number of 2-halo-N-arylacetamides 2 as shown in Table 2 were examined to broaden the scope of this protocol. In the presence of 2 equivalents of triethylamine as a base,the three-component one-pot reaction of potassium methyl cyanimidodithiocarbonate 1,2-halo-N-arylacetamides 2 and ethanol underwent readily,and thus producing the desired trisubstituted thiazoles in moderate to good yields (41–84%). It was indicated that the reaction could well tolerate the variations of electron-poor (compounds 4l–n) and electron-rich (compounds 4o–r) aromatic groups (Ar).
Preliminarily,the reaction mechanism for the formation of 2- alkoxy-4-amino-N-arylthiazole-5-carboxamides 4 was hypothesized using compound 4a as an example as shown in Scheme 2. It was reported that the reaction of potassium methyl cyanimidodithiocarbonate 1 with 2-chloro-N-(naphthalen-2-yl)-acetamide 2a could give rise to thiazolidone 6 [20] in ethanol in the absence of bases. And in this work,we isolated the intermediate 6 in 80% yield in ethanol and the intermediate 6 was heated at reflux in ethanol in the presence of triethylamine or NaH,and the desired compound 4a was obtained in 60% and 65% yield,respectively. In order to further improve the yield of this reaction,the following attempts were conducted. When sodium ethoxide was used as the base for the preparation of 4a with method A,the product 4a was isolated in only 36% yield. While when KI (1 equiv.) was used with 2- bromo-N-b-naphthylacetamide 2a or 2-iodo-N-b-naphthylacetamide 2j was utilized to promote this reaction,the desired compound 4a was obtained in comparable yield (57% and 52% yield,respectively),compared with the case where 2-bromo-N-bnaphthylacetamide 2a was treated as a reactant. Based on these experimental results,a plausible mechanism for this threecomponent one-pot synthesis of trisubstituted thiazoles was suggested as illustrated in Scheme 2. The initial SN2 substitution of thiolate 1 with 2-bromo-N-(naphthalen-2-yl)-acetamide and the subsequent intramolecular cyclization of intermediate 5 yielded thiazolidone 6. Next,in the presence of triethylamine, ethanol attacks on the electrophilic carbon of N-cyanamide 6, followed by the intramolecular ring opening of 7 to provide the key intermediate 8. After deprotonation of 8 with Et3N,the generated 9 underwent an intramolecular cyclization by attacking the present cyano group to afford 10,which was tautomerized into the desired compound 4a with the assistance of Et3N. 4. Conclusion
An efficient and straightforward approach was developed for the construction of novel 2-alkoxy-4-amino-N-arylthiazole-5- carboxamide derivatives. It has been demonstrated that structurally diverse alcohols and 2-halo-N-arylacetamides,which were easily accessed by the reaction of 2-chloroacetyl chloride and arylamines,were well tolerated. Therefore,this three-component one-pot protocol could be used to build up a structurally diversified thiazole library for the drug discovery and development. Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 81273380) and ‘‘863’’ Program of China (No. 2012AA020302). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.12.005.
| [1] | N. Siddiqui, S.K. Arya, W. Ahsan, B. Azad, Diverse biological activities of thiazoles: a retrospect, Int. J. Drug Dev. Res. 3 (2011) 55-67. |
| [2] | S.J. Kashyap, V.K. Garg, P.K. Sharma, et al., Thiazoles: having diverse biological activities, Med. Chem. Res. 21 (2012) 2123-2132. |
| [3] | N. Siddiqui, M.F. Arshad, W. Ahsan, M.S. Alam, Thiazoles: a valuable insight into the recent advances and biological activities, Int. J. Pharm. Sci. Rev. Res. 1 (2009) 136-143. |
| [4] | N.K. Shah, N.M. Shah, M.P. Patel, R.G. Patel, Synthesis, characterization and antimicrobial activity of some new biquinoline derivatives containing a thiazole moiety, Chin. Chem. Lett. 23 (2012) 454-457. |
| [5] | C.B. Guo, Z.F. Cai, Z.R. Guo, et al., Design, synthesis and in vitro evaluation of thiazole derivatives of ibuprofen as cyclooxygenase-2 inhibitors, Chin. Chem. Lett. 17 (2006) 325-328. |
| [6] | S.F. Cui, Y. Wang, J.S. Lu, G.L.V. Damu, C.H. Zhou, Recent advances in application of thiazole compounds, Sci. China: Chem. 42 (2012) 1105-1131. |
| [7] | U. Galm, E. Wendt-Pienkowski, L.Y. Wang, et al., Comparative analysis of the biosynthetic gene clusters and pathways for three structurally related antitumor antibiotics bleomycin, tallysomycin and zorbamycin, J. Nat. Prod. 74 (2011) 526-536. |
| [8] | J. Zhong, Muscarine, imidazole, oxazole, and thiazole alkaloids, Nat. Prod. Rep. 20 (2003) 584-605. |
| [9] | S. Carradori, D. Secci, A. Bolasco, et al., Synthesis and cytotoxicity of novel (thiazol-2-yl)hydrazine derivatives as promising anti-Candida agents, Eur. J. Med. Chem. 65 (2013) 102-111. |
| [10] | Y.J. Yang, Y.N. Yang, J.S. Jiang, et al., Synthesis and cytotoxic activity of heterocycle-substituted phthalimide derivatives, Chin. Chem. Lett. 21 (2010) 902-904. |
| [11] | J.D. Moore, A. Potter, Pin1 inhibitors: pitfalls, progress and cellular pharmacology, Bioorg. Med. Chem. Lett. 23 (2013) 4283-4291. |
| [12] | Y.Y. Xi, J. Jin, Y. Sun, et al., Design, synthesis and biological evaluation of novel diaryl ethers bearing a pyrimidine motif as human Pin1 inhibitors, Acta Pharm. Sin. 48 (2013) 1266-1272. |
| [13] | C. Liu, J. Jin, L. Chen, et al., Synthesis and biological evaluation of novel human Pin1 inhibitors with benzophenone skeleton, Bioorg.Med. Chem. 20 (2012) 2992-2999. |
| [14] | L.N. Zhu, J. Jin, C. Liu, et al., Synthesis and biological evaluation of novel quinazoline-derived human Pin1 inhibitors, Bioorg. Med. Chem. 19 (2011) 2797-2807. |
| [15] | C.J. Zhang, Z.H. Zhang, B.L. Xu, Y.L. Wang, Recent advances in the study of Pinl and its inhibitors, Acta Pharm. Sin. 43 (2008) 9-17. |
| [16] | T. Fuchigami, T. Nonaka, Facile synthesis of 5-acyl-4-amino-2-ethoxythiazoles from an [ethoxy(thiocarbonyl)]cyanamide salt and α-halo ketones, J. Org. Chem. 48 (1983) 3340-3341. |
| [17] | T. Fuchigami, M.Y. Yeh, T. Nonaka, H.J. Tien, Studies of cyanamide derivatives. Part 109. A convenient method for preparation of 4-aminothiazole compounds from alkoxythiocarbonylcyanamide salts, Bull. Chem. Soc. Jpn. 56 (1983) 3851-3852. |
| [18] | P. van der Peet, C.T. Gannon, I. Walker, et al., Use of click chemistry to define the substrate specificity of leishmania b-1,2-mannosyltransferases, ChemBioChem 7 (2006) 1384-1391. |
| [19] | Y. Jin, Z.Y. Zhou, W. Tian, Q. Yu, Y.Q. Long, 40-Alkoxyl substitution enhancing the anti-mitotic effect of 5-(3',4',5'-substituted)anilino-4-hydroxy-8-nitroquinazolines as a novel class of anti-microtubule agents, Bioorg. Med. Chem. Lett. 16 (2006) 5864-5869. |
| [20] | D. Wobig, Thiazolderivate, Ⅲ. Reaktionen von cyanimidodithiocarbonaten mit achloracetamiden, Liebigs Ann. Chem. 1977 (1977) 400-406. |
| [21] | The synthetic procedure and structure identification for compounds 2a-j, 4a-i and 4k-r are available in Supporting information. All synthesized final products were characterized by 1H NMR, 13C NMR and ESI-HRMS. For example, compound 4a: light yellow solid; mp 179-182 ℃; 1H NMR (300 MHz, DMSO-d6): δ 10.77 (s, 1H), 8.27 (s, 1H), 7.79-7.89 (m, 3H), 7.58 (dd, 1H, J1 = 8.7 Hz, J2 = 2.1 Hz), 7.48 (t, 1H, J = 6.9 Hz), 7.38 (t, 1H, J = 7.2 Hz), 6.95 (brs, 2H), 4.14 (q, 2H, J = 6.9 Hz), 1.22 (t, 3H, J = 6.9 Hz); 13C NMR (100 MHz, DMSO-d6): δ 165.04, 163.47, 137.56, 133.60, 129.41, 128.73, 127.53, 127.02, 126.61, 124.44, 119.59, 113.80, 58.97, 14.65; HRMS (ESI) Calcd. for C16H16N3O2S [M+H+]: 314.0958; found: 314.0957. |