b State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071, China;
c School of Materials and Chemical Engineering, Chongqing University of Arts and Science, Chongqing 402160, China
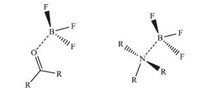
Boron trifluoride,as a typical electron deficient Lewis acid, exhibits markedly strong interactions with numerous electron donors [1]. In most cases,it can form complexes with nitrogen and oxygen moieties with strong Lewis basicities such as R2CO...BF3 and R3N...BF3,in Fig. 1. Among these strong electron donor/ acceptor complexes,their interaction energies have been investigated quantitatively [2] along with the BF3 affinity scale [3] and they play an essential role in many catalytic applications in organic chemistry,such as alkylation [4],acylation [5] and cyclization [6].
|
Download:
|
| Fig. 1.Structures of R2CO...BF3 and R3N...BF3 complexes | |
Compounds with an indole ring possess plentiful bioactivities and pharmacological activities [7],such as cytotoxic [8],and antifungal properties [9] in inhibiting the growth of tumor cells [10]. B is(indolyl)methanes containing important indole ring units are useful intermediates and can be utilized in interesting transformations of indole chemistry [11]. Great efforts have been made to seek efficient and convenient synthetic routes of bis(indolyl)methanes and therefore over the past few years, bis(indolyl)methanes have been synthesized by electrophilic substitution of indoles with carbonyl compounds catalyzed by Lewis acids,such as Ln(OTf)3 [12],GaCl3 [13],ZnO [14],FeCl3.6H2O [15],Al2O3 [16],zirconyldodecylsulfate [17],I2 [18],silica gel [19], pyridinium tribromide [20] and SiO2/AlCl3 [21]. Protic acids,such as montmorillonite clay [22, 23],oxone [24],NH4Cl [25],cellulose sulfuric acid [26],ion liquid [27] and oxalic acid [28] were also utilized to promote these transformations. Moreover,Schiff base– Cu(II) complex [29] and surfactant SLES [30] were proved to be effective for the synthesis of bis(indolyl)methanes. Based on the understanding of strong electron donor/acceptor interaction patterns between carbonyl/amine and BF3 [31],it is highly anticipated that this transformation would be accelerated in the presence of a catalytic amount of BF3.Et2O. In previous work,we have reported on Cs2CO3-catalyzed transesterification,primary amine-catalyzed aldol condensation,theoretical halo-exchange reactions,iodination of sp3 C–H with hypervalent iodide,and Lewis reactions,iodination of sp3 C–H with hypervalent iodide,and Lewis acid-promoted oxidative coupling of anilines [32]. Herein we describe an efficient and practical method for the synthesis of bis(indolyl)methanes between indoles and a series of carbonyl compounds at room temperature catalyzed by BF3.Et2O,which is attractive due to low cost,high catalytic efficiency and metal-free profile. 2. Experimental
BF3.Et2O (21.3 mg,0.15 mmol) was added to a mixture of indole 1 (234.4 mg,2 mmol) and benzaldehyde 2a (106.1 mg,1 mmol) in diethyl ether (2 mL). The reaction mixture was stirred at room temperature for 2 h in the atmosphere and monitored by TLC. After completion of the reaction,the volatile components were removedusing a vacuum rotary evaporator. The resulting residue was purified by column chromatography on silica gel column using EtOAc/petroleum ether solution (1:15–1:8,v/v) as eluent to afford the desired product bis(indolyl)phenylmethane 3a. Yield: 290 mg (90%); pink solid. 1H NMR (500 MHz,CDCl3): δ 7.91 (s,2H),7.39 (d, 2H,J = 8.0 Hz),7.36 (d,2H,J = 8.0 Hz),7.35 (d,2H,J = 7.0 Hz),7.28 (t,2H,J = 7.0 Hz),7.22–7.19 (m,1H),7.17 (t,2H,J = 7.0 Hz),7.00 (t, 2H,J = 7.5 Hz),6.66 (d,2H,J = 1.5 Hz),5.89 (s,1H). 13C NMR (125 MHz,CDCl3): δ 144.2,136.9,128.9,128.4,127.2,126.3,123.8, 122.1,120.1,119.9,119.4,111.2,40.4.
For detailed experimental procedure,crystallographic data, characterization data of the products,and copies of 1H NMR and 13C NMR,see Supporting information. 3. Results and discussion
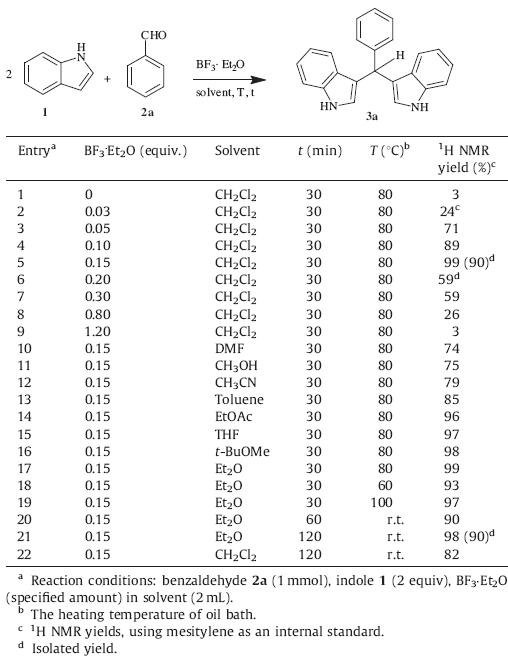
Initially,we aimed at determining the optimal reaction conditions for the synthesis of bis(indolyl)methanes (Table 1). The treatment of indole 1 with benzaldehyde 2a was used as a model reaction to screen various amount of catalysts,diverse solvents,and different reaction times and temperatures. The yields of 3a were determined by1HNMRspectroscopy usingmesitylene as aninternal standard.When the amount of BF3.Et2Owas increased to 0.15 equiv, increasing yields of 3a were obtained with 0.15 equiv of BF3.Et2O resulting in a 99% yield (entries 1–5). Nevertheless,further increases in the amount of BF3.Et2O resulted in dramatically decreasing yields (entries 6–9). Performances in different solvents were scanned (entries 10–17),eventually confirming that diethyl ether afforded a comparative yield (entry 17). Higher temperatures were tolerated for this reaction (entries 17–19). In an effort to get amilder reaction condition,studies with prolonged reaction times at room temperature were tried and,to our delight,an excellent yield was provided after 2 h in diethyl ether (entry 21),while dichloromethane only led to lower yield of 82% (entry 22). Therefore,the following optimal reaction conditionswere established: aldehyde 2a (1mmol),indole 1 (2 equiv),and BF3.Et2O (0.15 equiv) in Et2O (2mL) at room temperature for 2 h.
| Table 1 Optimization of reaction conditions. |
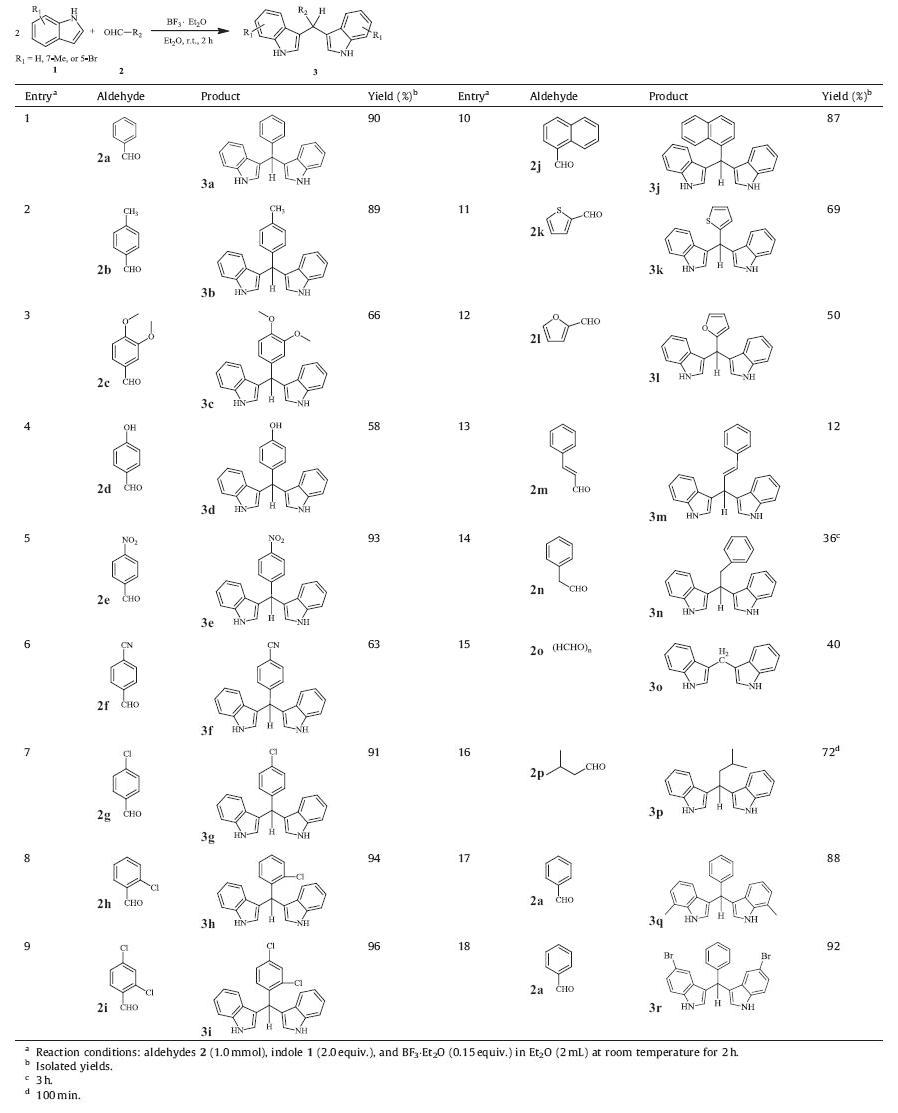
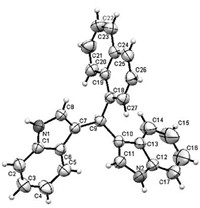
With the optimal conditions in hand,we set about expanding the scope of aldehydes (Table 2). Aromatic,heteroaromatic,fused, aliphatic and enal aldehydes are suitable substrates to synthesize bis(indolyl)methanes with moderate to excellent yields. Aromatic aldehydes with neutral,electron-donating and electron-withdrawing groups on the phenyl ringwere tolerated.Methyl (2b),nitro (2e), p-chloro (2g),o-chloro (2h) and 2,4-dichloro (2i) substituents produced the corresponding products in excellent yields of above 89%. Benzaldehydes with oxygen-bearing functional groups,such as methoxyl (2c) and hydroxy (2d),gave somewhat lower yields, presumably due to the complexation of oxygen with BF3.Et2O. Cyanobenzaldehyde 2f with a versatile cyano group in known organic transformation produced 3f in 63% yield. Reaction of naphthaldehyde 2j gave 3j which also occurred in good yield of 87% (entry 10)withthe structure of 3j further confirmed by single crystal X-ray crystallography (Fig. 2) [33]. Heteroaromatic aldehydes,such as thiophenecarboxaldehyde 2k and furfural 2l provided moderate yields (entries 11 and 12). Cinnamyl aldehyde 2m was also tested, and only a low yield was obtained (entries 13). Aliphatic aldehydes, such as phenylacetaldehyde 2n,formaldehyde 2o and isovaleraldehyde 2p afforded the corresponding products 3n,3o and 3p in 36%,40% and 72% yields,respectively (entries 14–16). The reactions of 7-methyl-1H-indole and 5-bromo-1H-indole were also examined and the corresponding products 3q and 3r were obtained in good yields of 88% and 92%,respectively.
| Table 2 Reactions of indole with aldehydes catalyzed by BF3.Et2O. |
|
Download:
|
| Fig. 2.X-ray single crystal structure of 3j. | |
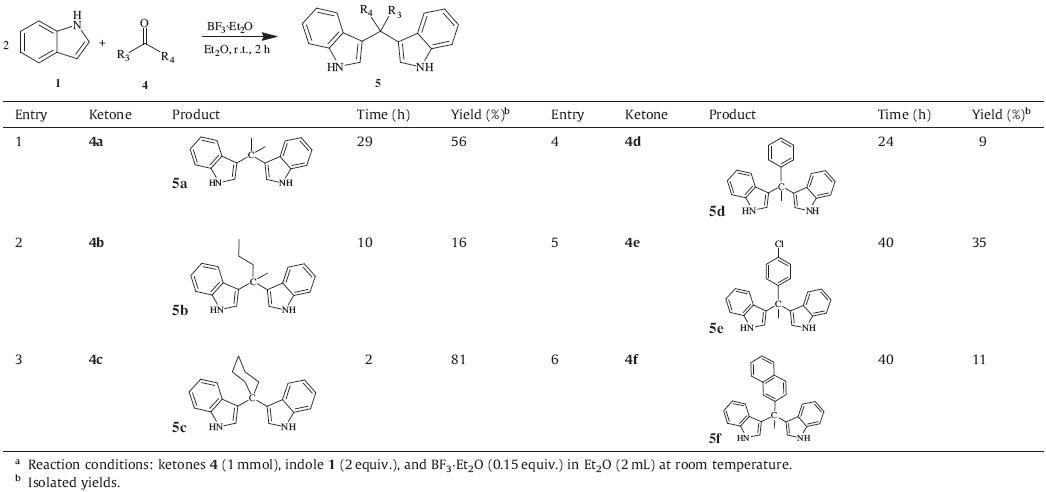
Furthermore,the less reactive ketonic substrates 4a–f were chosen to evaluate this methodology with indoles (Table 3). Aliphatic ketones such as acetone 4a and 2-pentanone 4b were examined and gave moderate yields over 24 h. It is noteworthy that cyclohexanone provided the target product 5c in 81% yield. In the case of aromatic ketones 5d–f,yields of 9%,35% and 11% were obtained,respectively.
| Table 3 Reactions of indole with ketones catalyzed by BF3.Et2O. |
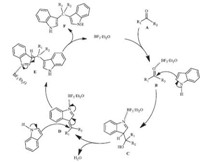
Based on the features of BF3.Et2O,catalyzed reactions with BF3.Et2O were carried out under mild reaction conditions,and the mechanism in Fig. 3was proposed. The initial step of themechanism is the interaction between BF3.Et2O and analog A to form the complex B,as in the R2CO...BF3 complex,and then a molecule of indole attacks B to generate intermediate C,as in the R3N...BF3 complex. Through the strong interaction between nitrogen and BF3.Et2O in C,an elimination of a molecule H2O is accelerated,and intermediate C is transformed into D. Subsequently,another molecule indole is reacted with D via an aza-Michael addition to afford complex E. With a molecule BF3.Et2O released,the bis(indolyl)methane F is produced,and the catalyst BF3.Et2O then enters another catalytic cycle.
|
Download:
|
| Fig. 3.A proposed mechanism for the synthesis of indoles with different carbonyl compounds. | |
To sum up,a strategy for using BF3.Et2O as an efficient catalyst for electrophilic substitution reactions of indoles with carbonyl compounds has been developed as anticipated,which gives structurally diverse bis(indolyl)methanes with yields up to 96%. The mild and metal-free reaction conditions render this methodology a practical protocol. A possible mechanism is proposed to interpret the observed reactivities. Acknowledgments
We are grateful for scientific research fundings from the Scientific Research Foundation for the Returned Overseas Chinese Scholars,State Education Ministry,Partenariats Hubert Curien Xu Guangqi 2012 (No. 27967RE),Fundamental Research Funds for the Central Universities (Nos. CDJRC10220004 and CDJZR11220005), and Natural Science Foundation Project of CQ CSTC (Nos. 2010BB5064 and cstc2013jcyjA0217) for financial support. Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2013. 11.038.
| [1] | K. Ishihara, Lewis Acids in Organic Synthesis, Wiley-VCH, Weinheim, 2008p. 96. |
| [2] | H. Umeyama, K. Morokuma, Molecular orbital studies of electron donor-acceptor complexes. 3. Energy and charge decomposition analyses for several strong complexes: OC-BH3, H3N-BH3, CH3H2N-BH3, (CH3)3N-BH3, and H3N-BF3, J. Am. Chem. Soc. 98 (1976) 7208-7220. |
| [3] | C. Laurence, J.F. Gal, Lewis Basicity and Affinity Scales: Data and Measurement, John Wiley & Sons, Wiltshire, 2010p. 85. |
| [4] | G.K.S. Prakash, F. Paknia, T. Mathew, et al., Fluoroanalogs of DDT: superacidic BF3-H2O catalyzed facile synthesis of 1,1,1-trifluoro-2,2-diarylethanes and 1,1-difluoro-2,2-diarylethanes, Org. Lett. 13 (2011) 4128-4131. |
| [5] | J. Zakrzewski, M. Karpińska, Z. Maliński, A large scale synthesis of a natural antibiotic 2,4-diacetylophloroglucinol (DAPG), Arch. Pharm. Chem. Life Sci. 340 (2007) 103-106. |
| [6] | (a) N.C. Ma, K.M. Wu, L. Huang, An interesting cyclization of N-methyl-3-phenyl-N-(2-(Z)-phenylethenyl)-cis-oxiranecarboxamide, J. Heterocycl. Chem. 45 (2008) 785-787; (b) D.J. Xiao, L.J. Wang, X.M. Feng, A practical synthetic pathway to polysubstituted tetrahydropyridines via multicomponent reactions catalyzed by BF3 OEt2, Synlett 10 (2005) 1531-1540. |
| [7] | B. Xu, Z.L. Guo, W.Y. Jin, et al., Multistep one-pot synthesis of enantioenriched polysubstituted cyclopenta[b]indoles, Angew. Chem. Int. Ed. 51 (2012) 1059-1062. |
| [8] | B.Q. Bao, Q.S. Sun, X.S. Yao, et al., Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp., J. Nat. Prod. 68 (2005) 711-715. |
| [9] | S. Sakemit, H.H. Sun, Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge spongosorites ruetzleri, J. Org. Chem. 56 (1991) 4304-4307. |
| [10] | (a) S. Tsujii, K.L. Rinehart, S.P. Gunasekera, et al., Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: antiviral and antitumor bis(indoly1)imidazoles from caribbean deep-sea sponges of the family halichondriidae. Structural and synthetic studies, J. Org. Chem. 53 (1988) 5446-5453; (b) C.M. Cover, S.J. Hsieh, S.H. Tran, et al., Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling, J. Biol. Chem. 273 (1998) 3838-3847; (c) S. Safe, S. Papineni, S. Chintharlapalli, Cancer chemotherapy with indole-3-carbinol, bis(30-indolyl)methane and synthetic analogs, Cancer Lett. 269 (2008) 326-338; (d) T.P. Pathak, J.G. Osiak, R.M. Vaden, B.E. Welm, M.S. Sigman, Synthesis and preliminary biological study of bisindolylmethanes accessed by an acid-catalyzed hydroarylation of vinyl indoles, Tetrahedron 68 (2012) 5203-5215. |
| [11] | M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, Bis-and trisindolylmethanes (BIMs and TIMs), Chem. Rev. 110 (2010) 2250-2293. |
| [12] | D. Chen, L. Yu, P.G. Wang, Lewis acid-catalyzed reactions in protic media. Lanthanide-catalyzed reactions of indoles with aldehydes or ketones, Tetrahedron Lett. 37 (1996) 4467-4470. |
| [13] | J.S. Yadav, B.V.S. Reddy, B. Padmavani, M.K. Gupta, Gallium(Ⅲ) halide-catalyzed coupling of indoles with phenylacetylene: synthesis of bis(indolyl)phenylethanes, Tetrahedron Lett. 45 (2004) 7577-7579. |
| [14] | M. Hosseini-Sarvari, Synthesis of bis(indolyl)methanes using a catalytic amount of ZnO under solvent-free conditions, Synth. Commun. 38 (2008) 832-840. |
| [15] | S.J. Ji, M.F. Zhou, D.G. Gu, Z.Q. Jiang, T.P. Loh, Efficient FeⅢ-catalyzed synthesis of bis(indolyl)methanes in ionic liquids, Eur. J. Org. Chem. 2004 (2004) 1584-1587. |
| [16] | S.A. Sadaphal, A.H. Kategaonkar, V.B. Labade, M.S. Shingare, Synthesis of bis(indolyl) methanes using aluminium oxide (acidic) in dry media, Chin. Chem. Lett. 21 (2010) 39-42. |
| [17] | M. Jafarpour, A. Rezaeifard, T. Golshani, A new catalytic method for ecofriendly synthesis of bis-and trisindolylmethanes by zirconyldodecylsulfate under mild conditions, J. Heterocycl. Chem. 46 (2009) 535-539. |
| [18] | (a) B.P. Bandgar, K.A. Shaikh, Molecular iodine-catalyzed efficient and highly rapid synthesis of bis(indolyl)methanes under mild conditions, Tetrahedron Lett. 44 (2003) 1959-1961; (b) S.J. Ji, S.Y. Wang, Y. Zhang, T.P. Loh, Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions, Tetrahedron 60 (2004) 2051-2055. |
| [19] | S.R. Mendes, S. Thurow, M.P. Fortes, et al., Synthesis of bis(indolyl)methanes using silica gel as an efficient and recyclable surface, Tetrahedron Lett. 53 (2012) 5402-5406. |
| [20] | Q. Yang, Z.L. Yin, B.L. Ouyang, Y.Y. Peng, Pyridinium tribromide catalyzed condensation of indoles and aldehydes to form bisindolylalkanes, Chin. Chem. Lett. 22 (2011) 515-518. |
| [21] | K.P. Boroujeni, K. Parvanak, Efficient and solvent-free synthesis of bis-indolylmethanes using silica gel supported aluminium chloride as a reusable catalyst, Chin. Chem. Lett. 22 (2011) 939-942. |
| [22] | M. Chakrabarty, N. Ghosh, R. Basaka, Y. Harigaya, Dry reaction of indoles with carbonyl compounds on montmorillonite K10 clay: a mild, expedient synthesis of diindolylalkanes and vibrindole A, Tetrahedron Lett. 43 (2002) 4075-4078. |
| [23] | J.S. Yadav, B.V.S. Reddy, G. Satheesh, Montmorillonite clay catalyzed alkylation of pyrroles and indoles with cyclic hemi-acetals, Tetrahedron Lett. 45 (2004) 3673-3676. |
| [24] | C.L. Zhang, Z.Q. Du, Synthesis of bis-indolylmethanes catalyzed by oxone, Chin. Chem. Lett. 20 (2009) 1411-1414. |
| [25] | J. Azizian, F. Teimouri, M.R. Mohamadizadeh, Ammonium chloride catalyzed onepot synthesis of diindolylmethanes under solvent-free conditions, Catal. Commun. 8 (2007) 1117-1121. |
| [26] | H. Alinezhad, A.H. Haghighi, F. Salehian, A green method for the synthesis of bisindolylmethanes and 3,3'-indolyloxindole derivatives using cellulose sulfuric acid under solvent-free conditions, Chin. Chem. Lett. 21 (2010) 183-186. |
| [27] | P.J. Das, J. Das, Synthesis of aryl/alkyl(2,2'-bis-3-methylindolyl)methanes and aryl(3,3'-bis indolyl)methanes promoted by secondary amine based ionic liquids and microwave irradiation, Tetrahedron Lett. 53 (2012) 4718-4720. |
| [28] | N.D. Kokare, J.N. Sangshetti, D.B. Shinde, Oxalic acid as a catalyst for efficient synthesis of bis-(indolyl)methanes, and 14-aryl-14H-dibenzo[a,j]xanthenes in water, Chin. Chem. Lett. 19 (2008) 1186-1189. |
| [29] | Y.L. Yang, N.N. Wan, W.P. Wang, Z.F. Xie, J.D. Wang, Synthesis of bis(indolyl) methanes catalyzed by Schiff base-Cu(Ⅱ) complex, Chin. Chem. Lett. 22 (2011) 1071-1074. |
| [30] | E. Kolvari, M.A. Zolfigol, H. Banary, Surfactant-assisted synthesis of bis(indolyl)-methanes in water, Chin. Chem. Lett. 22 (2011) 1305-1308. |
| [31] | A. Chatterjee, S. Manna, J. Bawrji, et al., Lewis-acid-induced electrophilic substitution in indoles with acetone. Part 2, J. Chem. Soc., Perkin Trans. 1 (1980) 553-555. |
| [32] | (a) S.Y. Zheng, Y. Xiong, J.Y. Wang, Theoretical studies on identity SN2 reactions of lithium halide and methyl halide: a microhydration model, J. Mol. Model. 16 (2010) 1931-1937; (b) Y. Xiong, X.Q. Zhang, Significant heterogeneous carbonate salt catalyzed acetylation of alcohols via a transesterification process with carbonate saltactivated alcohol 1H NMR evidence, Chin. J. Chem. 29 (2011) 1143-1148; (c) Y. Xiong, S.T. Zhang, X.G. Ling, X. Zhang, J.Y. Wang, Theoretical investigation on identical anionic halide-exchange SN2 reaction processes on N-haloammonium cation NH3X+ (X = F, Cl, Br, and I), Int. J. Quantum Chem. 112 (2012) 2475-2481; (d) X. Zhang, Y. Xiong, S.T. Zhang, et al., Aldol condensations of aldehydes and ketones catalyzed by primary amine on water, Asian J. Chem. 24 (2012) 751-755; (e) X.G. Ling, Y. Xiong, S.T. Zhang, R.F. Huang, X.H. Zhang, Effective synthesis of benzyl halides triggered by in situ prepared hypervalent halides, Chin. Chem. Lett. 24 (2013) 45-48; (f) X.G. Ling, Y. Xiong, R.F. Huang, et al., Synthesis of benzidine derivatives via FeCl3 6H2O-promoted oxidative coupling of anilines, J. Org. Chem. 78 (2013) 5218-5226. |
| [33] | The crystal data of compound 3j (CCDC reference number 946729): C27H20N2, M = 372.46, crystal system: monoclinic, space group: P21/c, lattice parameters: a = 10.722(6)Å, b = 15.124(6)Å, c = 15.355(9)Å, α = 90°, β = 105.159(11)°, γ = 90°, V = 2406(2)Å3, Z = 4, Dc = 1.244 g/cm-3, F000 = 952, final R indices[I > 2sigma(I)]: R1 = 0.0670, wR2 = 0.2023. |