Among a large diversity of heterocyclic compounds,bridgehead hydrazine heterocycles have attracted considerable attention because they show some pharmacological properties and biological activities [1–5]. Among which,pyrazole derivatives are acknowledged to possess a wide range of bioactivities and exhibit important biological properties such as anti-inflammatory [6], antifungal [7],anticancer [8],antiviral [9],antitumor [10], anticoagulant [11] antibacterial (inhibitory activity against Escherichia coli FabH) [12],and antihypoglycemic activity [13, 14]. Phthalazine derivatives were reported to possess a multiplicity of pharmacological properties including antimicrobial [15], anticonvulsant [16],antifungal [17],anticancer [18],cardiotonic [19],vasorelaxant [20] activities. Pyrazolo[1,2-d]phthalazinedione derivatives have been described as anti-inflammatory, analgesic,anti-hypoxic,and anti-pyretic agents [21] and therefore, the development of simple methods for the synthesis of pyrazolo[1,2-d]phthalazine-5,10-diones is considered very important.
Multi-component reactions (MCRs) constitute a very powerful tool to synthesize diverse and complex heterocyclic compounds. Meanwhile,more classical drug-like,heterocyclic core structures can be created through MCR synthesis [22, 23, 24, 25, 26]. Consequently,MCRs have been paid much attention by synthetic organic chemists worldwide due to the effectiveness of multiple component reactions at building functionalized,drug-likes structures from different families of compounds in a single step and thus we believe that discovering and developing new and novel ones is an important pursuit in academic chemistry [27, 28].
Similarly nanoparticles have undergone extensive examination in the past decade. Recent advances in nanoscience and nanotechnology have led to new research interests in using nanometer-sized particles as an alternative matrix for catalytic reactions. Among various nanoparticles,copper nanoparticles have received considerable attention because of their unique properties and potential applications in diverse fields [29]. Recently,copper nanoparticles were used as a suitable catalyst in many reactions including synthesis of alkyne-azide cycloadditions [30],the Mannich reaction [31],aza-Michael reactions [32],hydroxylation of phenol [33],1,4-dihydropyridines [34],polyfunctionalized pyridine derivatives [35],and functionalized tricarboxamides [36].
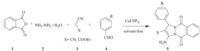
The synthesis of 1H-pyrazolo[1,2-d]phthalazine-5,10-dione derivatives via the three-component coupling of aldehydes,malononitrile or ethyl cyanoacetate and phthalhydrazide has been reported using MCRs in the presence of diverse catalysts including PTSA/[Bmim]Br (100°C,3 h) [37],Et3N/EtOH (50°C,1 h,sonochemistry) [38],[Bmim]OH/MW (100 W,45°C,4–5 min) [39],1,8- diazabicyclo[5, 4, 0]-undec-7-en-8-ium acetate,(DBU[CH3COO], (solvent-free) [40],AL-KIT-6(ethanol,60°C,4 h) [41],NiCl2.6H2O (ethanol/reflux) [42] InCl3 [43]. Herein,we report the use of CuI nanoparticles as an efficient catalyst for the preparation of 1H-pyrazolo[1,2-d]phthalazine- 5,10-diones by the four-component condensation reactions of phthalic anhydride,hydrazine monohydrate,aromatic aldehydes and malononitrile or ethyl cyanoacetate under solvent-free conditions in good to excellent yields (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of 1H-pyrazolo[1,2- b]phthalazine-5,10-dione derivatives. | |
The CuI nanoparticles were prepared according to the procedure reported in the literature [44]. Where the catalyst was prepared by ultrasonic irradiation approach. Firstly,the copper substrate (CuSO4) (1 mmol) is cleaned ultrasonically for 20 s in acetone and then in a 2 mol/L HCl solution,followed by repeated rinsing with distilled water. After drying,the substrate is dipped slowly into a solution of KI (2 mmol) in 40 mL of distilled water, sonicated and allowed to react for 30 min. When the reaction was completed,a gray precipitate was obtained. The solid was filtered and washed with distilled water,ethanol and dried at room temperature for 48 h. 2.2. General procedure for the preparation of 1H-pyrazolo[1,2- b]phthalazine-5,10-dione derivatives
Hydrazine monohydrate (1 mmol) and phthalic anhydride (1 mmol) were mixed at 70°C (15 min). Then,aromatic aldehydes (1 mmol),malononitrile or ethyl cyanoacetate (1 mmol) and CuI nanoparticles (10% mol),as catalyst,was added and stirred at 70 8C under solvent-free conditions for the specific time (Table 1). After completion of the reaction,the reaction mixture was recrystallized from MeOH to afford the pure product.
3-Amino-1-(2-methylphenyl)-5,10-dihydro-5,10-dioxo-1Hpyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5a): Yellow powder; mp 247–249°C,IR (KBr,cm-1):νmax 3383,3300,2207,1659,1603; 1H NMR (400 MHz,DMSO-d6): δ 2.43 (3H,s,CH3),6.30 (1H,s,CH), 7.17–7.28 (4H,m,Ar),7.96–8.25 (6H,m,Ar and NH2); 13C NMR (100 MHz,DMSO-d6): δ 18.65,61.2,62.5,122.0,126.8,127.2,127.8, 128.0,128.3,128.5,128.7,130.5,133.8,134.8,135.2,136.5,153.7, 154.2,156.6. Anal. Calcd. for C19H14N4O2: C 69.09,H 4.27,N 16.95, Found: C 68.98,H 4.25,N 16.98,MS (EI) (m/z): 330.
3-Amino-1-(3-methylphenyl)-5,10-dihydro-5,10-dioxo-1Hpyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5b): Yellow powder; mp 250–252°C,IR (KBr,cm-1): νmax 3361,3259,2194,1658,1570; 1H NMR (400 MHz,DMSO-d6): δ 2.27 (3H,s,CH3),6.05 (1H,s,CH), 7.12–7.24 (4H,m,Ar),7.96–8.26 (6H,m,Ar and NH2); 13C NMR (100 MHz,DMSO-d6): δ 24.0,61.3,63.2,122.1,122.3,127.2,127.4, 127.8,128.2,128.6,128.9,131.4,134.0,134.6,135.6,136.3,154.0, 154.5,157.3. Anal. Calcd. for C19H14N4O2: C 69.09,H 4.27,N 16.95, Found: C 69.15,H 4.15,N 16.87,MS (EI) (m/z): 330.
3-Amino-1-(4-methylphenyl)-5,10-dihydro-5,10-dioxo-1Hpyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5c): Yellow powder; mp253–255°C,IR (KBr,cm-1): νmax 3361,3259,2196,1656,1569; 1H NMR (400 MHz,DMSO-d6): δ 2.28 (3H,s,CH3),6.07 (1H,s,CH), 7.14–7.33 (4H,m,Ar),7.94–8.25 (6H,m,Ar and NH2); 13C NMR (100 MHz,DMSO-d6): δ 21.2,62.3,64.0,123.1,1234,127.4,127.6, 127.9,128.1,128.7,130.2,130.8,133.3,134.6,135.0,136.5,153.0, 154.7,157.0. Anal. Calcd. for C19H14N4O2: C 69.09,H 4.27,N 16.95, Found: C 69.11,H 4.19,N 16.90,MS (EI) (m/z): 330.
3-Amino-1-(4-isopropylphenyl)-5,10-dihydro-5,10-dioxo-1Hpyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5d): Yellow powder; mp265–267 8C,IR (KBr,cm-1): νmax 3368,3264,2191,1659,1569; 1H NMR (400 MHz,DMSO-d6): δ 1.18 (6H,d,J = 6.8 Hz),2.92 (1H, m),6.09 (1H,s,CH),7.23–7.35 (4H,m,Ar),7.95–8.24 (6H,m,Ar and NH2); 13C NMR (100 MHz,DMSO-d6): d 24.3,33.6,61.3,63.4,116.1, 126.9,127.32,127.7,129.1,133.6,134.21,148.4,150.6,153.4, 156.8. Anal. Calcd. for C21H18N4O2: C 70.36,H 5.06,N 15.63,Found: C 70.29,H 4.99,N 15.67,MS (EI) (m/z): 358.
3-Amino-1-(4-hydroxyphenyl)-5,10-dihydro-5,10-dioxo-1Hpyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5e): Yellow powder; mp 270–272°C,IR (KBr,cm-1):νmax 3372,3259,2197,1663,1568; 1H NMR (400 MHz,DMSO-d6): δ 6.02 (1H,s),6.70–6.72 (2H,d, J = 8 Hz,Ar),7.22–7.24 (2H,d,J = 8 Hz,Ar),7.95–8.24 (6H,m,Ar and NH2),9.51 (1H,s,OH); 13C NMR (100 MHz,DMSO-d6): d 61.4, 62.6,115.0,116.2,126.6,127.3,128.3,128.6,128.8,133.6,134.5, 150.5,153.5,156.6,157.6. Anal. Calcd. for C18H12N4O3: C 65.06,H 3.64,N 16.85,Found: C 64.98,H 3.57,N 16.80,MS (EI) (m/z): 332. 3-Amino-1-(4-chlorophenyl)-5,10-dihydro-5,10-dioxo-1H-pyrazolo[ 1,2-b]phthalazine-2-carbonitrile (5g): Yellow powder; mp 270–272 8C,IR (KBr,cm-1): νmax 3375,3259,2194,1659,1655; 1H NMR (400MHz,DMSO-d6): d 6.14 (1H,s,CH),7.39–7.52 (4H,m,Ar), 7.94–8.26 (6H,m,Ar and NH2); 13C NMR (100 MHz,DMSO-d6): d 61.3,62.8,116.3,127.2,127.7,128.8,129.3,133.3,134.3,135.1, 137.9,151.2,154.1,157.2. Anal. Calcd. for C18H11N4O2Cl: C 61.64, H 3.16,N 15.97,Found: C 61.51,H 3.13,N 16.01,MS (EI) (m/z): 350. Ethyl 3-amino-5,10-dihydro-1(4-isopropylphenyl)-5,10-dioxo- 1H-pyrazolo[1,2-d]phthalazine-2-carboxylate (5i): Yellow powder; mp 231–233 8C,IR (KBr,cm-1): νmax 3432,3324,1699,1665,1530; 1H NMR (400MHz,DMSO-d6): δ 1.00 (t,3H,J = 8 Hz,CH3),1.14 (d, 6H,J = 8 Hz,2CH3),2.69–2.82 (m,1H,CH),3.97 (m,2H,CH2),6.03 (s, 1H),7.13–7.36 (4H,m,Ar),7.94–8.25 (6H,m); 13C NMR (100 MHz, DMSO-d6): δ 14.1,23.8,33.0,58.4,62.9,81.7,125.6,126.6,127.2, 128.6,128.8,133.5,134.5,137.1,147.6,153.0,156.7,164.3. Anal. Calcd. for C23H23N3O4: C 68.14,H 5.71,N 10.36,Found: C 68.10,H 5.67,N 10.29,MS (EI) (m/z): 405. 3. Results and discussion
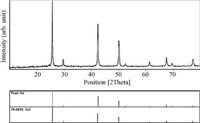
The morphology and particle size of copper(I) iodide nanoparticles, was investigated by scanning electron microscopy (SEM) before use and after reuse of three times with images shown in Fig. 1. The SEM images show particles with diameters in the range of nanometers. The XRD pattern of the CuI nanoparticles is shown in Fig. 2. The results show that spherical CuI nanoparticles were obtained with an average diameter of 10–30 nm as confirmed by XRD analysis.

|
Download:
|
| Fig. 1.SEM images of CuI NPs before use (a),after reuse of three times (b). | |
|
Download:
|
| Fig. 2.The XRD pattern of CuI(I) nanoparticles (NP). | |
Initially,we had explored and optimized different reaction parameters for the synthesis of 1H-pyrazolo[1,2-b]phthalazine- 5,10-diones by the four-component condensation reaction of phthalic anhydride,hydrazine monohydrate,benzaldehyde and malononitrile as a model reaction. Several reactions were scrutinized using different catalysts and various solvents,such as ethanol,acetonitrile,water,dichloromethane and solvent-free conditions (Tables 1 and 2). Under solvent-free conditions,we determined the best level for the synthesis of 1H-pyrazolo[1,2- b]phthalazine-5,10-dione derivatives was using CuI nanoparticles at 10 mol% which gave excellent yields of product. When 5,10 and 15 mol% of CuI nanoparticles were used,the yields were 82%,92% and 90%,respectively. Therefore,10 mol% of CuI nanoparticle was suitable and an excessive amount of catalyst did not increase the yields significantly. The effect of temperature was also evaluated for the model reaction (Table 3). Almost all reactions worked well with a variety of aromatic aldehydes and the desired compounds were obtained in good to high yields within short reaction time (Table 4).
| Table 1 The model reaction was carried out by various catalystsa |
| Table 2 The test reaction using different solvents with CuI nanoparticles.a |
| Table 3 Effects of temperature variation on the yield and time of the model reaction.a |
| Table 4 Synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones.a |
To optimize the experiment temperature,the mixture was heated at different temperatures ranging from 30°C to 80°C (Table 3). The time of reaction was decreased when the reaction temperature was raised from 30°C to 70°C. Therefore,70°C was chosen as the reaction temperature for all subsequent reactions.
All the reactions reached completion within 25–35 min and afforded good yields of products. As represented in Table 4,all aldehydes gave the expected products at high yields,either bearing electron-withdrawing groups or electron-donating groups under solvent-free conditions. 4. Conclusion
In this research,we described an efficient and one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones by the four-component condensation reaction of phthalic anhydride,hydrazine monohydrate,aromatic aldehydes and malononitrile or ethyl cyanoacetate under solvent-free conditions. The advantages offered by this method include,short reaction times,excellent yields,a simple procedure,easy workup and the employment of a cost-effective catalyst. Acknowledgment
The authors are grateful to University of Kashan for supporting this work (No. 159196/XIIV).
| [1] | W.R. Vaughan, The chemistry of the phthalazines, Chem. Rev. 43 (1948) 447-508. |
| [2] | H.W. Heine, R. Henrie, L. Heitz, S.R. Kovvali, Diaziridines Ⅲ. Reactions of some 1-alkyl-and 1,1-dialkyl-1H-diazirino[1,2-b]phthalazine-3,8-diones, J. Org. Chem. 39 (1974) 3187-3191. |
| [3] | S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian, M. Davoodnejad, A novel and efficient synthesis of pyrazolo[3,4-d]pyrimidine derivatives and the study of their anti-bacterial activity, Chin. Chem. Lett. 24 (2013) 629-632. |
| [4] | N.K. Terrett, A.S. Bell, D. Brown, P. Ellis, Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5cGMP, phosphodiesterase with utility for the treatment of maleerectile dysfunction, Bioorg. Med. Chem. Lett. 6 (1996) 1819-1824. |
| [5] | S.K. Singh, P.G. Reddy, K.S. Rao, et al., Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors, Bioorg. Med. Chem. Lett. 14 (2004) 499-504. |
| [6] | T. Nakamura, M. Sato, H. Kakinuma, et al., Pyrazole and isoxazole derivatives as new, potent, and selective 20-hydroxy-5,8,11,14-eicosatetraenoic acid synthase inhibitors, J. Med. Chem. 46 (2003) 5416-5427. |
| [7] | O. Prakash, R. Kumar, V. Parkash, Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl)chromones, Eur. J. Med. Chem. 43 (2008) 435-440. |
| [8] | M.A.F. Vera-DiVaio, A.C.C. Freitas, H.C.A. Castro, et al., Synthesis, antichagasic in vitor evaluation, cytotoxicity assays, molecular modeling and SAR/QSAR studies of a 2-phenyl-3-(1-phenyl-1H-pyrazol-4-yl) acrylic acid benzylidene-carbohydrazide series, Bioorg. Med. Chem. 17 (2009) 295-302. |
| [9] | M.J. Genin, C. Biles, B.J. Keiser, et al., Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: lead identification and SAR of 3-and 4-substituted derivatives, J. Med. Chem. 43 (2000) 1034-1040. |
| [10] | F. Wei, B.X. Zhao, B. Huang, et al., Design, synthesis, and preliminary biological evaluation of novel ethyl 1-(2'-hydroxy-3'-aroxypropyl)-3-aryl-1H-pyrazole-5-carboxylate, Bioorg, Med. Chem. Lett. 16 (2006) 6342-6347. |
| [11] | Y. Xia, Z.W. Dong, B.X. Zhao, et al., Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells, Bioorg. Med. Chem. 15 (2007) 6893-6899. |
| [12] | P.C. Lü, J. Sun, Y. Luo, Y. Yang, H.L. Zhu, Design, synthesis, and structure activity relationships of pyrazole derivatives as potential FabH inhibitors, Bioorg. Med. Chem. Lett. 20 (2010) 4657-4660. |
| [13] | N. Cho, M. Kamaura, T. Yogo, H. Imoto, Preparation of pyrazole derivatives as improvement of insulin resistance, WO 2009139340. |
| [14] | K. Dugi, M. Mark, F. Himmelsbach, Pharmaceutical composition comprising a pyrazole-O-glucoside derivative, WO 2009022009 A 1. |
| [15] | S.S. El-Saka, A.H. Soliman, A.M. Imam, Synthesis, antimicrobial activity and electron impact of mass spectra of phthalazine-1,4-dione derivatives, Afinidad 66 (2009) 167-172. |
| [16] | L. Zhang, L.P. Guan, X.Y. Sun, et al., Synthesis and anticonvulsant activity of 6-alkoxy-[1,2,4]triazolo[3,4-a]phthalazines, Chem. Biol. Drug Des. 73 (2009) 313-319. |
| [17] | C.K. Ryu, R.E. Park, M.Y. Ma, J.H. Nho, Synthesis and antifungal activity of 6-arylamino-phthalazine-5,8-diones and 6,7-bis(arylthio)-phthalazine-5,8-diones, Bioorg. Med. Chem. Lett. 17 (2007) 2577-2580. |
| [18] | J. Li, Y.F. Zhao, X.Y. Yuan, J.X. Xu, P. Gong, Synthesis and anticancer activities of novel 1,4-disubstituted phthalazines, Molecules 11 (2006) 574-582. |
| [19] | Y. Nomoto, H. Obase, H. Takai, et al., Studies on cardiotonic agents. Ⅱ. Synthesis of novel phthalazine and 1,2,3-benzotriazine derivatives, Chem. Pharm. Bull. (Tokyo) 38 (1990) 2179-2183. |
| [20] | N. Watanabe, Y. Kabasawa, Y. Takase, et al., 4-Benzylamino-1-chloro-6-substituted phthalazines: synthesis and inhibitory activity toward phosphodiesterase 5, J. Med. Chem. 41 (1998) 3367-3372. |
| [21] | F. Al'-Assar, K.N. Zelenin, E.E. Lesiovskaya, I.P. Bezhan, B.A. Chakchir, Synthesis and pharmacological activity of 1-hydroxy, 1-amino-, and 1-hydrazino-substituted 2,3-dihydro-1H-pyrazolo[1,2-a]pyridazine-5,8-diones and 2,3-dihydro-1H-pyrazolo[1,2-b]phthalazine-5,10-diones, J. Pharm. Chem. 36 (2002) 598-603. |
| [22] | J. Sinkkonen, V. Ovcharenko, K.N. Zelenin, et al., 1H and 13C NMR study of 1-hydrazino-2,3-dihydro-1H-pyrazolo[1,2-a]pyridazine-5,8-diones and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones and their ring-chain tautomerism, Eur. J. Org. Chem. 13 (2002) 2046-2053. |
| [23] | R.P. Jain, J.C. Vederas, Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase, Bioorg. Med. Chem. Lett. 14 (2004) 3655-3658. |
| [24] | A. Kumar, M.K. Gupta, M. Kumar, L-Proline catalysed multicomponent synthesis of 3-amino alkylated indoles via a Mannich-type reaction under solvent-free conditions, Green Chem. 14 (2012) 290-295. |
| [25] | I. Ugi, A. Dömling, W. Hörl, Multicomponent reactions in organic chemistry, Endeavour 18 (1994) 115-122. |
| [26] | M.M. Heravi, B. Baghernejad, H.A. Oskooie, A novel three-component reaction for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amines, Tetrahedron Lett. 50 (2009) 767-769. |
| [27] | J. Gerencser, G. Dormon, F. Darvas, Meldrum's acid in multicomponent reaction: applications to combinatorial and diversity-oriented synthesis, QSAR Comb. Sci. 25 (2006) 439-448. |
| [28] | D.J. Ramon, M. Yus, Asymmetric multicomponent reactions (AMCRs): the new frontier, Angew. Chem. Int. Ed. 44 (2005) 1602-1634. |
| [29] | L. Lu, M.L. Sui, K. Lu, Superplastic extensibility of nanocrystalline copper at room temperature, Science 287 (2000) 1463-1466. |
| [30] | Y.J. Song, C. Yoo, J.T. Hong, et al., Nanocrystalline copper oxide(Ⅱ)-catalyzed alkyne-azide cycloadditions, Bull. Korean Chem. Soc. 29 (2008) 1561-1564. |
| [31] | M. Kidwai, N.K. Mishra, V. Bansal, A. Kuma, S. Mozumdar, Novel one-pot Cunanoparticles-catalyzed Mannich reaction, Tetrahedron Lett. 50 (2009) 1355-1358. |
| [32] | A.K. Verma, R. Kumar, P. Chaudhary, et al., Cu-nanoparticles: a chemoselective catalyst for the aza-Michael reactions of N-alkyl and N-arylpiperazines with acrylonitrile, Tetrahedron Lett. 46 (2005) 5229-5232. |
| [33] | A.K. Edward, L.M. Anton, S.K. Yulia, et al., Copper nanoparticles as active catalysts in hydroxylation of phenol by hydrogen peroxide, Appl. Catal. A: Gen. 385 (2010) 62-72. |
| [34] | J. Safaei-Ghomi, A. Ziarati, R. Teymuri, CuI nanoparticles as new, efficient and reusable catalyst for the one-pot synthesis of 1,4-dihydropyridines, Bull. Korean Chem. Soc. 33 (2012) 2679-2682. |
| [35] | J. Safaei-Ghomi, M.A. Ghasemzadeh, CuI nanoparticles: a highly active and easily recyclable catalyst for the synthesis of 2-amino-3,5-dicyano-6-sulfanyl pyridines, J. Sulfur Chem. 34 (2013) 233-241. |
| [36] | A. Ziarati, J. Safaei-Ghomi, S. Rohani, Pseudo five-component process for the synthesis of functionalized tricarboxamides using CuI nanoparticles as reusable catalyst, Chin. Chem. Lett. 24 (2013) 195-198. |
| [37] | R. Ghahremanzadeh, G. Imani Shakibaei, A. Bazgir, An efficient one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dionederivatives,Synlett8 (2008)1129-1132. |
| [38] | M.R. Nabid, S.J. Tabatabaei Rezaei, R. Ghahremanzadeh, A. Bazgir, Ultrasoundassisted one-pot, three-component synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, Ultrason. Sonochem. 17 (2010) 159-161. |
| [39] | D.S. Raghuvanshi, K.N. Singh, A highly efficient green synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives and their photophysical studies, Tetrahedron Lett. 52 (2011) 5702-5705. |
| [40] | H.R. Shaterian, M. Mohammadnia, Mild basic ionic liquids catalyzed new fourcomponent synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, J. Mol. Liq. 173 (2012) 55-61. |
| [41] | G. Karthikeyan, A. Pandurangan, Post synthesis alumination of KIT-6 materials with la3d symmetry and their catalytic efficiency towards multicomponent synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione carbonitriles and carboxylates, J. Mol. Catal. A: Chem. 361-362 (2012) 58-67. |
| [42] | S.H. Song, J. Zhong, Y.H. He, Z. Guan, One-pot four-component synthesis of 1Hpyrazolo[1,2-b]phthalazine-5,10-dione derivatives, Tetrahedron Lett. 53 (2012) 7075-7077. |
| [43] | M. Veeranarayana Reddy, Y. Tae Jeeong, InCl3-catalyzed green synthesis of 1Hpyrazolo[1,2-b]phthalazine-5,10-diones under solvent-free conditions, Tetrahedron Lett. 54 (2013) 3546-3549. |
| [44] | Y. Jiang, S.Y. Gao, Z.D. Li, X.X. Jia, Y.L. Chen, Cauliflower-like CuI nanostructures: green synthesis and applications as catalyst and adsorbent, Mater. Sci. Eng. B 176 (2011) 1021-1027. |
| [45] | A. Azarifar, R. Nejat-Yami, D. Azarifar, Nano-ZnO: an efficient and reusable catalyst for one-pot synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones and pyrazolo[1,2-a][1,2,4]triazole-1,3-diones, J. Iran. Chem. Soc. 10 (2013) 297-306. |