Alkylboronic acid derivatives are interesting compounds in medicinal chemistry [1]. In synthetic organic chemistry alkylboronic acid derivatives are often employed as reagents for transition metal-catalyzed cross-coupling reactions of C(sp3) organometallics [2]. The development of new methods to synthesize alkylboronic acid derivatives has been intensively studied in synthetic organic chemistry. Recent attention has been given to the development of transition metal-catalyzed preparation of alkylboronic acid derivatives. Some important examples include Fe-,Rh- and Ir-catalyzed hydroboration of alkenes [3],Cu-, Pt-,Pd-,and Rh-catalyzed boration of unsaturated carbonyl compounds [4],and Ru-,Rh-,Pd-,Ir-,and Re-catalyzed C(sp3)-H activation/borylation of alkanes [5].
Very recently,several groups developed a new approach for the synthesis of alkylboronic acid derivatives through Cu-,Pd-,and Nicatalyzed borylation of alkyl halides and pseudohalides [6]. These reactions provide a useful new strategy for the preparation of alkylboronic acids with stereochemical controls beyond those of the traditional methods. Beyond these pioneering examples, further development of borylation of alkyl halides (particularly those using alternative transition metal catalysts) is needed to expand the scope and utility of this category of synthetically valuable transformations. Herein,we describe the first example of rhodium-catalyzed borylation of unactivated alkyl chlorides. This method expands the concept and scope of rhodium-catalyzed cross-coupling reactions [7, 8] in a fundamental way. 2. Experimental
1H,11B and 13CNMRspectra were recorded on a Bruker Advance 400 spectrometer. Gas chromatographic (GC) analysis was acquired on a Shimadzu GC-2014 Series GC System equipped with a flameionization detector. HRMS analysis was performed on Finnigan LCQ advantage Max Series MS System. Commercially obtained reagents were used without further purification. All reactions were monitored by TLC silica gel coated plates. Column chromatography was carried out using 300–400 mesh silica gel at medium pressure. 2.1. General procedure for alkylboronic esters
In air,[Rh(COD)Cl]2 (2.5 mg,0.005 mmol),terpyridine (2.73 mg, 0.01 mmol),t-BuONa (48 mg,0.5 mmol),and bis(pinacolato)- diboron (95 mg,0.375 mmol) were added to a Schlenk tube equipped with a stir bar. The vessel was evacuated and filled with argon (three cycles). t-AmylOH (1.0 mL) and alkyl chloride (0.25 mmol) were added sequentially under an argon atmosphere. The resulting reaction mixture was stirred vigorously at 80&lamdba;C for 24 h. Then water was added to the mixture at room temperature. The resulting mixture was extracted with ethyl acetate three times, and the combined organic phase was washed with water and brine and then dried over MgSO4. After filtration and evaporation of the solvent,the crude mixture was purified by column chromatography on silica gel with petroleum ether/ethyl acetate to give the target product. 2.2. Selected data of compound 3
4,4,5,5-Tetramethyl-2-(3-phenoxypropyl)-1,3,2-dioxaborolane (3b): White solid. 1H NMR (400 MHz,CDCl3): δ7.29–7.23 (m,2H), 6.91 (m,3H),3.94 (t,2H,J = 6.7 Hz),1.99–1.83 (m,2H),1.25 (s, 12H),0.92 (t,2H,J = 7.8 Hz). 13C NMR (101 MHz,CDCl3): δ159.18, 129.36,120.38,114.61,83.10,69.53,24.84,23.79.
4,4,5,5-Tetramethyl-2-(3-(naphthalen-2-yloxy)propyl)-1,3,2- dioxaborolane (3c): White solid. 1H NMR (400 MHz,CDCl3): δ 7.78–7.68 (m,3H),7.41 (t,1H,J = 7.5 Hz),7.31 (t,1H,J = 7.5 Hz), 7.14 (d,2H,J = 7.3 Hz),4.06 (t,2H,J = 6.7 Hz),2.02–1.89 (m,2H), 1.26 (s,12H),0.97 (t,2H,J = 7.8 Hz). 13C NMR (101 MHz,CDCl3): δ 156.09,133.60,128.20,127.81,126.58,125.65,125.19,122.36, 118.10,105.59,82.10,68.60,23.82,22.69. HRMS calcd. for C19H25BO3 (M+): 312.1897; found: 312.1891
4,4,5,5-Tetramethyl-2-phenethyl-1,3,2-dioxaborolane (3e): Colorless liquid. 1H NMR (400 MHz,CDCl3): δ7.24 (m,4H), 7.17–7.09 (m,1H),2.79–2.68 (m,2H),1.21 (s,12H),1.18–1.09 (m, 2H). 13C NMR (101 MHz,CDCl3): δ 143.39,127.15,126.98,124.46, 82.06,28.92,23.78.
Trimethyl(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- propyl)silane (3g): Colorless liquid. 1H NMR (400 MHz,CDCl3): d 1.49–1.38 (m,2H),1.24 (s,12H),0.83 (t,2H,J = 7.6 Hz),0.57–0.45 (m,2H),-0.03 (s,9H). 13C NMR (101 MHz,CDCl3): δ81.79,23.82, 19.09,17.55,-2.63.
1-(6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)hexyl)-1Hindole (3i): White solid. 1H NMR (400 MHz,CDCl3): δ 7.61 (d,1H, J = 7.9 Hz),7.32 (d,1H,J = 8.2 Hz),7.18 (t,1H,J = 7.6 Hz),7.08 (t,2H, J = 5.8 Hz),6.50–6.44 (m,1H),4.08 (t,2H,J = 7.2 Hz),1.88–1.76 (m, 2H),1.46–1.36 (m,2H),1.30 (d,4H,J = 14.1 Hz),1.22 (s,12H),0.75 (t,2H,J = 7.4 Hz). 13C NMR (101 MHz,CDCl3): δ135.95,128.56, 127.76,121.24,120.89,119.09,109.37,100.79,82.88,46.33,31.88, 30.12,26.74,24.81,23.84. 3. Results and discussion
Our study began with the borylation of 1 by B2Pin2 (2) (Table 1). When [Rh(COD)Cl]2 was used as the catalyst,we tested different ligands (entries 1–5). Fortunately,we observed 67% yield of the desired product by using terpridine as ligand (entries 2). Other ligands (1,10-phen,IMesHCl,S-Binap) were examined,but they produced lower yields,while no products were obtained in the absence of a ligand. By changing the reaction temperatures we further improved the yield to 85% at 80°C. Lower yieldwas obtained when the reactionwas conducted at 60°Cor100°C (entries 6 and 8). Subsequently we tested different solvents (entries 9–12),but the yields were lower. Other alkyl halides were also examined (entries 13 and 14),with 67% yields and 35% yields of the desired product by using the corresponding alkyl bromide and alkyl iodide. Furthermore, a control experiment confirmed that the reaction does not take place without [Rh(COD)Cl]2 (entry 15).
| Table 1 Oligonucleotides designed in the present study.a |
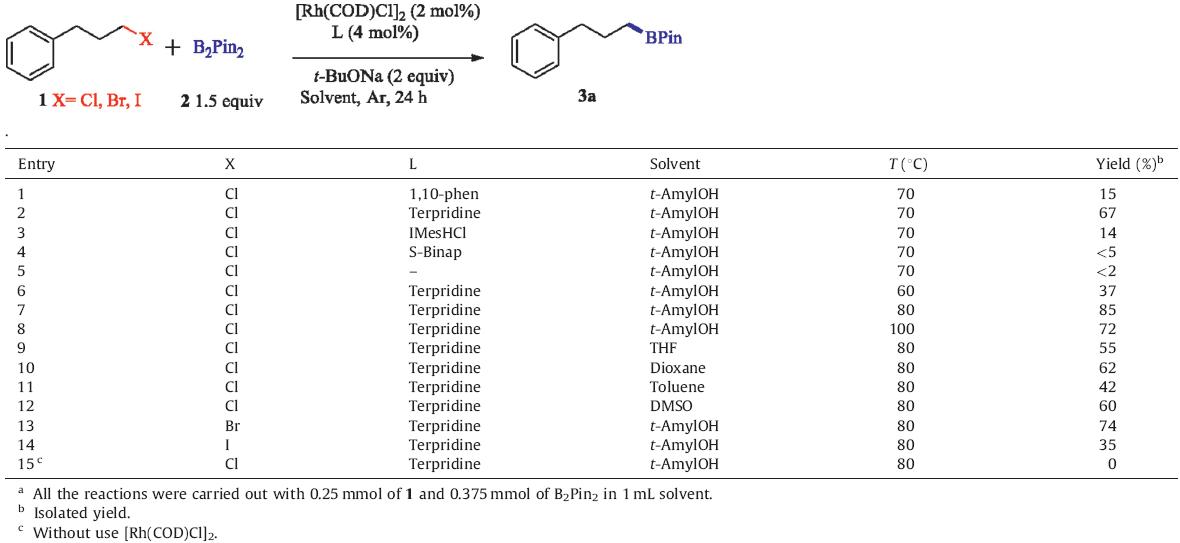
Having optimized the conditions,we next examined the scope of the Rh(I)-catalyzed borylation reaction (Fig. 1). Our results show that synthetically important functional groups including ether (3b, 3c,3d),ester (3h),or silane (3g) can be well tolerated in the reaction. We have confirmed that aryl chlorides are in fact less reactive than alkyl chlorides (3d). This allows chloro-substituted arene ring to be used successfully,thus providing the potential for subsequent modifications through additional cross-coupling reactions at the halogenated positions. Moreover,heterocycles which are highly interesting building blocks in the drug design, such as indole (3i),amide (3j),and chromenone (3k) are also compatible with the borylation conditions. Nonetheless,the present conditions cannot be used to second alkyl chloride (3l) [9].
|
Download:
|
| Fig. 1.Scope of Rh-catalyzed borylation. The reactions were conducted under the optimized conditions on a 0.25 mmol scale. Isolated yields are reported. | |
Weproposed that the mechanism of the Rh-catalyzed borylation is as follows: First,the reaction of [Rh(COD)Cl]2,ligand andt-BuONa with diboron reagent generates borylrhodiumA. Note that recently the Chatani group reported the Rh(I)-catalyzed borylation of nitriles, where borylrhodium complex is proposed to be the active catalyst [10]. Second,oxidative addition of alkyl chloridewith borylrhodium A takes place to produce B [11]. Finally,reductive elimination takes place from B to generate the target product.
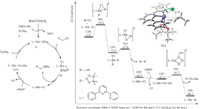
Density functional theory calculations were conducted to study the mechanism [12]. As shown in Fig. 2,oxidative addition of alkyl chloride occurs on borylrhodium complex CP0 through transition state TS1 to afford complex CP1. The energy barrier for oxidative addition is +29.0 kcal/mol. Then rapid reduction of CP1 occurs through TS2 to obtain the borylation product and complex CP2. CP2 exchanges the anionic ligand to form CP3. CP3 reacts with diboron reagent through TS3 to regenerate the catalyst CP0 with the aid of the nucleophilic attack of tert-butoxide ion. In the catalytic cycle oxidative addition of alkyl halides is the ratedetermining step.
|
Download:
|
| Fig. 2.Propsed mechanism and corresponding theoretical calculations | |
In conclusion,we have developed Rh-catalyzed cross-coupling reactions of unactivated alkyl chlorides with diboron reagents. These reactions can be used to prepare primary alkylboronic esters with diverse skeletons and functional groups. Theoretical analysis indicates that the reaction may proceed through an oxidative addition of alkyl chlorines on a borylrhodium complex. This transformation expands the concept and scope of rhodiumcatalyzed cross-coupling reactions in a fundamental sense. Acknowledgments
This study was supported by the National Basic Research Program of China (973 Program; No. 2013CB932800),NSFC (No. 20972148),and CAS (No. KJCX2-EW-J02). We also thank the support of China Postdoctoral Science Foundation (Nos. 2011M500289,2012T50078). Related theoretical study was supported by ChinaGrid project funded by MOE of China and Supercomputer Center of USTC.
| [1] | M.A. Beenen, C. An, J.A. Ellman, Asymmetric copper-catalyzed synthesis of aamino boronate esters from N-tert-butanesulfinyl aldimines, J. Am. Chem. Soc. 130 (2008) 6910-6911. |
| [2] | (a) R. Jana, T.P. Pathak, M.S. Sigman, Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl organo-metallics as reaction partners, Chem. Rev. 111 (2011) 1417-1492; (b) C.T. Yang, Z.Q. Zhang, Y.C. Liu, L. Liu, Copper-catalyzed cross coupling reaction of organoboron compounds with primary alkyl halides and pseudohalides, Angew. Chem. Int. Ed. 50 (2011) 3904-3907. |
| [3] | (a) D.A. Evans, G.C. Fu, A.H. Hoveyda, Rhodium(I)-and iridium(I)-catalyzed hydroboration reactions: scope and synthetic applications, J. Am. Chem. Soc. 114 (1992) 6671-6679; (b) L. Zhang, D. Peng, X. Leng, Z. Huang, Iron-catalyzed, atom-economical, chemoand regioselective alkene hydroboration with pinacolborane, Chem. Int. Ed. 52 (2013) 3676-3680. |
| [4] | (a) M.O. Brien, K.S. Lee, A.H. Hoveyda, Enantioselective synthesis of boronsubstituted quaternary carbons by NHC-Cu-catalyzed boronate conjugate additions to unsaturated carboxylic esters, ketones, or thioesters, J. Am. Chem. Soc. 132 (2010) 10630-10633; (b) Y. Sasaki, Y. Horita, C. Zhong, M. Sawamura, H. Ito, Copper(I)-catalyzed regioselective monoborylation of 1,3-enynes with an internal triple bond: selective synthesis of 1,3-dienylboronates and 3-alkynylboronates, Angew. Chem. Int. Ed. 50 (2011) 2778; (c) J.A. Schiffner, K. Muethner, M. Oestreich, Enantioselective conjugate borylation, Angew. Chem. Int. Ed. 49 (2010) 1194-1196; (d) E. Hartmann, D.J. Vyas, M. Oestreich, Enantioselective formal hydration of a,bunsaturated acceptors: asymmetric conjugate addition of silicon and boron nucleophiles, Chem. Commun. 47 (2011) 7917-7932. |
| [5] | (a) H. Chen, S. Schlecht, T.C. Semple, J.F. Hartwig, Thermal, catalytic, regiospecific functionalization of alkanes, Science 287 (2000) 1995-1997; (b) H. Chen, J.F. Hartwig, Catalytic, regiospecific end-functionalization of alkanes: rhenium-catalyzed borylation under photochemical conditions, Angew. Chem. Int. Ed. 38 (1999) 3391-3393; (c) S. Shimada, A.S. Batsanov, J.A.K. Howard, T.B. Marder, Formation of aryl-and benzylboronate esters by rhodium-catalyzed C-H bond functionalization with pinacolborane, Angew. Chem. Int. Ed. 40 (2001) 2168; (d) J.D. Lawrence, M. Takahashi, C. Bae, J.F. Hartwig, Regiospecific functionalization of methyl C-H bonds of alkyl groups in reagents with heteroatom functionality, J. Am. Chem. Soc. 126 (2004) 15334-15335; (e) J.M. Murphy, J.D. Lawrence, K. Kawamura, C. In-carvito, J.F. Hartwig, Ruthenium-catalyzed regiospecific borylation of methyl C-H Bonds, J. Am. Chem. Soc. 128 (2006) 13684-13685;(f) C.S. Wei, C.A. Jiménez-Hoyos, M.F. Videa, J.F. Hartwig, M.B. Hall, Origins of the selectivity for borylation of primary over secondary C-H bonds catalyzed by Cp*-rhodium complexes, J. Am. Chem. Soc. 132 (2010) 3078-3091; (g) S. Kawamorita, T. Miyazaki, T. Iwai, H. Ohmiya, M. Saw, Rh-catalyzed borylation of N-adjacent C(sp3)-H bonds with a silica-supported triarylphosphine ligand, J. Am. Chem. Soc. 134 (2012) 12924-12927; (h) S. Kawamorita, R. Murakami, T. Iwai, M. Sawamura, Synthesis of primary and secondary alkylboronates through site-selective C(sp3)-H activation with silica-supported monophosphine-Ir catalysts, J. Am. Chem. Soc. 135 (2013) 2947-2950. |
| [6] | (a) C.T. Yang, Z.Q. Zhang, H. Tajuddin, et al., Alkylboronic esters from coppercatalyzed borylation of primary and secondary alkyl halides and pseudohalides, Angew. Chem. Int. Ed. 51 (2012) 528-532; (b) H. Ito, K. Kubota, Copper(I)-catalyzed boryl substitution of unactivated alkyl halides, Org. Lett. 14 (2012) 890-893; (c) J. Yi, J.H. Liu, J. Liang, et al., Alkylboronic esters from palladium-and nickelcatalyzed borylation of primary and secondary alkyl bromides, Adv. Synth. Catal. 354 (2012) 1685-1691; (d) A.S. Dudnik, G.C. Fu, Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon-boron bonds, J. Am. Chem. Soc. 134 (2012) 10693-10697; (e) A. Joshi-Pangu, X. Ma, M. Diane, et al., Palladium-catalyzed borylation of primary alkyl bromides, J. Org. Chem. 77 (2012) 6629-6633; (f) K. Kubota, E. Yamamoto, H. Ito, Copper(I)-catalyzed borylativeexo-cyclization of alkenyl halides containing unactivated double bond, J. Am. Chem. Soc. 135 (2013) 2635-2640; (g) M. Presset, N. Fleury-Bré geot, D. Oehlrich, F. Rombouts, G.A. Molander, Synthesis and minisci reactions of organotrifluoroborato building blocks, J. Org. Chem. 78 (2013) 4615-4619; (h) H. Li, L. Wang, Y. Zhang, J. Wang, Transition-metal-free synthesis of pinacol alkylboronates from tosylhydrazones, Angew. Chem. Int. Ed. 51 (2012) 2943-2946. |
| [7] | S. Ejiri, S. Odo, H. Takahashi, et al., Alkyl-aryl cross-coupling catalyzed by Rh: efficiency of novel tripodal 3-diphenylphosphino-2(diphenylphosphino)-methyl-2-methylpropyl acetate Ligand, Org. Lett. 12 (2010) 1692-1695. |
| [8] | (a) T.J. Gong, B. Xiao, Z.J. Liu, et al., Rhodium-catalyzed selective C-H activation/olefination of phenol carbamates, Org. Lett. 13 (2011) 3235-3237; (b) X.J.Du,Y.H.Tang,X.Zhang,M. Lei,Atheoretical studyonthe alkeneinsertionstep in Rh-Yanphos catalyzed hydroformylation, Chin. Chem. Lett. 24 (2013) 1083-1086; (c) Z.J. Ji, J.Y. Jiang, Y.H. Wang, A novel thermoregulated phosphine ligand used for the Rh-catalyzed hydroformylation of mixed C11-12 olefins in aqueous/organic biphasic system, Chin. Chem. Lett. 21 (2010) 515-518. |
| [9] | C.T. Yang, Z.Q. Zhang, J. Liang, et al., Copper-catalyzed cross-coupling of nonactivated secondary alkyl halides and tosylates with secondary alkyl grignard reagents, J. Am. Chem. Soc. 134 (2012) 11124-11127. |
| [10] | M. Tobisu, H. Kinuta, Y. Kita, E. Rémond, N. Chatani, Rhodium(I)-catalyzed borylation of nitriles through the cleavage of carbon-cyano bonds, J. Am. Chem. Soc. 134 (2012) 115-118. |
| [11] | B.C. de Pater, E.J. Zijp, H.W. Frü hauf, et al.Oxidative addition reactions of [RhI (Br)(Tpy*)] (Tpy* = 40-(4-ert-butylphenyl)-2,20:60,200-terpyridine) with alkyl bromides, Organometallics 23 (2004) 269-279. |
| [12] | Y.Y. Jiang,H.Z.Yu, Y.Fu,Mechanistic studyof borylation of nitriles catalyzed byRh-B and Ir-B complexes via C-CN bond activation, Organometallics 32 (2013) 926-936. |