Inspired by fantastic natural phenomena, stimuli–responsive systems were established and extensively developed in the past two decades. Among them, ever-increasing attention has been paid to the field of responsive polymers due to their adjustable molecular structure and polymorphism of morphologies [1–3], which can exhibit reversible or irreversible changes in physical properties and/or chemical structures in response to external stimuli, such as pH [4,5], temperature [6,7], ionic [8], light [1– 3,9,10] electric and magnetic fields [11,12], redox potential [13,14], CO2 [15,16] or a combination of them, to name a few [16,17]. Of these stimuli, light is an especially attractive external stimulus and has been used in various areas. The use of light to trigger a desired reaction of a polymer enables more temporal and positional control and have been frequently employed in specific applications, such as optically rewritable data storage [18], optical switching [19], chemical sensing [8], drug or enzyme delivery systems [20], smart surfaces [21], cell imaging [22] and bioseparation [23].
Anchoring photochromic groups into a polymer chain to enable its light-sensitive property is a common strategy to construct a light-responsive polymer. Upon light irradiation, the chemical (cross-linking, isomerization, etc.) or physical parameters (polarity, hydrophilicity, etc.) of the photochromic segment would be responsively altered, inducing the change of polymer conformations. Driven by the potential application in controlled drug delivery, the principles of amphiphilic block copolymer (BCP) design for photo-dissociable core–shell micelles or vesicles (polymersomes) have received increasing attention. This kind of light-responsive BCPs assembles can be regrouped into four types based on their photo-induced structural changes [24]. The first approach based on optically shifting the hydrophilic–hydrophobic balance of BCPs is the most studied one. The other three approaches are light induced breaking block junction, main chain degradation and reversible cross-linking. Azobenzenes [1,25], spiropyrans (SPs) [9,10,13,26], diazonaphthoquinone (DNQ) [27], o-nitrobenzyl [28] and coumarin [29] compounds are extensively studied photochromers. Compared to other photochromic molecules, spiropyrans are demandable photochromic compounds, which can easily and reversibly be inter-converted between ringclosed, colorless spiropyran (SP) and colored, ring-opened merocyanine (MC) forms with UV (365 nm) and visible light (550 nm), respectively. The SP and MC states have remarkably different chemical structures and physical properties. Consequently, with an incorporated spiropyran compound into polymer chain as a hydrophobic segment, the hydrophilic-hydrophobic balance of the copolymer would responsively be shifted with the isomerization of SP to MC. The polymer assembles were also process disrupted and regeneration. This property enables this functional copolymer to be an excellent light triggered delivery system for drugs. Because spiropyran has a light switchable fluorescence property, it can be used as a fluorescence probe at the same time.
In this work, amphiphilic copolymer, PEG-b-PSPMA, was synthesized via one step RAFT polymerization, as one of powerful methods to give well-defined polymers. A PEG based macro-chain transfer agent was prepared and used as the modulator to manipulated the polymerization of 1'-(2-methacryloxyethyl)- 3'3'-dimethyl-6-nitro-spiro(2H-1-benzo-pyran-2,2'-indoline) (SPMA). This SP-based copolymer was self-assembled into micelles, which can be disrupted by UV irradiation and reversibly regenerated by irradiating with visible light. The light switchable responsiveness was very fast and not very dependent on the photochromic concentration. Moreover, the copolymer containing grafted spiropyran displayed a photo-switchable, dual-color, fluorescence property in aqueous solution, which is suitable to image the cancer cell tissue during anti-cancer drug release. However, the fluorescence intensity of MC is so weak that it cannot be distinguished from background signals. To address this problem, a fluorescence resonance energy transfer (FRET) process was introduced to increase the fluorescence signal. Therefore, a hydrophobic fluorescent dye, nitrobenzoxadiazolyl derivative (NBD), a good FRET donor to MC, was encapsulated within the hydrophobic cavities of the polymeric micelles. The PEG-b-PSPMA copolymer can also reduce the cytotoxicity and incompatibility within aqueous media of NBD dye. Exposed to alternative UV/vis irradiation, the photo-switching between orange and red fluorescence of this system can be facilely achieved. These light trigger responsivities and photo-switchable FRET processes enable this copolymer to have a potential application in drug delivery and cell imaging or tracking synchronously.
Poly(ethylene glycol, Mn = 5000) (PEG), 4-chloro-7-nitrobenzofurazan (NBD-Cl, 99%), 2,3,3-trimethylindolenine (98%), 2-iodoethanol (99%) and 5-nitrosalicylaldehyde (98%) were purchased from Alfa and used as received. Methacryloyl chloride was purchased from TCI and storage at -10°C before use. S- 1-Dodecyl-S-(a,a'dimethyl-a"-acetic acid) trithiocarbonate (DBATC) was synthesized according to Ref. [30] in 40.6% yield. Monomer 10-(2-methacryloxyethyl)-3',3'-dimethyl-6-nitro-spiro (2H-1-benzopyran-2,2'-indoline) (SPMA) was synthesized by esterification of SPOH with methacryloyl chloride according to Ref. [31] with slight modification in 40% yield. 1H NMR (SPMA) (400 MHz, CDCl3): d 1.16 (–CH3), 1.28 (–CH3), 1.91 (–CH3), 3.37– 3.62 (–N–CH2–), 4.3 (–O–CH2–), 5.56 and 6.07 (=CH2), 5.87 (–CH=), 6.67–6.80 (Ar–H and –CH=), 6.86–6.95 (2Ar=H), 7.06– 7.13 (Ar–H), 7.17–7.24 (Ar–H), 7.97–8.05 (2Ar–H). Poly(ethylene glycol)-based chain transfer agent (PEG-CTA) was synthesized according to the literature procedure [32] in a yield of 80%. 1H NMR (400 MHz, DMSO-d6): d 0.9 (–CH3), 1.23 (–CH2–), 1.65 (–CH3), 3.42 (–CH2–S–), 3.28 (–O–CH3), 3.45–4.25 (–O–CH2–CH2–O–), 3.68 (–CO–O–CH2–CH2–), 4.5 (–CO–O–CH2–). 2,2'-Azobisisobutyronitrile (AIBN) was purified by recrystallization from ethanol, and then dried under vacuum. The water used in this work was double distilled water, which was further purified with a Milli-Q system. Tetrahydrofuran (THF, A.R.), dichloromethane (DCM, A.R.) and triethylamine (A.R.) were distilled over CaH2 and then distilled just before use. All other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. and used as received.
The 1H NMR spectra were recorded in CDCl3 and DMSO-d6 solution, on a Bruker spectrometer operating at a frequency of 400 MHz for protons. The molecular weight and molecular distribution measurements were performed using a Waters GPC device with THF as eluent at a flow rate of 0.6 mL min-1 at 40°C. UV/vis absorption was acquired on a VARIAN Cary 50 Probe spectrophotometer. The measurements of fluorescence spectra were carried out on a VARIAN Cary Eclipse luminescence spectrometer at room temperature. TEM observations were conducted on a Tecnai G2 F20 electron microscope (FEI Co.) at an acceleration voltage of 80 kV. The average size and size distribution of micelles in aqueous solution were determined by dynamic light scattering (DLS) after filtrated through a 0.45 mm Millipore filter. The measurements were carried out on a Brookhaven model BI-200SM spectrometer and 9000AT correlator using an Innova304 He–Ne laser (1 W, λ= 532 nm) at a fixed scattering angle (u) of 90° for during of 2 min. The temperature was set at 25°C. For irradiation experiments, the samples were irradiated with UV light at 365 nm, or visible light at 550 nm. The UV and visible light source is a 300W high-pressure Xe lamp (CEL-HXUV300, AULTT, Co., China).
A typical procedure of RAFT polymerization is as follows: Into a 10 mL polymerization tube, PEG-CTA (0.047 mmol, 0.25 g), SPMA (4.7 mmol, 2 g), AIBN (0.048 mmol, 8 mg) and THF (5 mL) were added. After bubbled N2 for 30 min, the tube was sealed and immersed into an oil bath thermostated at 75°C. The polymerizations were conducted 24 h and 48 h, respectively, and after which the tubes were cooled to room temperature rapidly, and the polymer was obtained after re-precipitation three times from diethyl ether. The resulting product was dried overnight in a vacuum oven at 40°C temperature. (Mn,GPC = 6435, Mw/Mn = 1.22, SP moiety was 9 for 24 h sample and Mn,GPC = 9617, Mw/Mn = 1.52, SP moiety was 18 for 48 h sample).
PEG-b-PSPMA (Mn,GPC = 6435, 10 mg) was dissolved in THF (3 mL). Under vigorously stirring, deionized water was added slowly. Then the dispersion was slowly stirred for another 5 h and THF was removed by dialysis [molecular weight (Mw) cut off: 3000 Da] against deionized water for 48 h and then diluted to a preset concentration. The solution was filtered through a 0.45 mm filter before use. The critical micellization concentrations (CMC) of the copolymers were determined on a luminescence spectrometer with pyrene as fluorescent probe at a fixed exciting wavelength (λ) of 334 nm. Complex PEG-b-PSPMA core–shell micelles encapsulated with NDB dyes were prepared by a similar procedure.
Similar to the common thermo RAFT polymerization procedure, the synthetic strategy in this research is schematically illustrated in Scheme 1. The macro RAFT agent, PEG-CTA, was synthesized by esterification of mPEG with DBATC, a well known trithiocarbonate chain transfer agent used in RAFT polymerization, and catalyzed by DCC and DMAP. The successful esterification was unambiguously demonstrated by the disappearance of the characteristic resonance peak of the proton of carboxyl, and, at the same time, the observed appearance of the peaks at d 4.5 and d 3.45–4.25, attributed to the protons of –CO–O–CH2– and PEG segment, respectively, as illustrated in Fig. 1. The light-sensitive monomer, SPMA was polymerized under RAFT conditions manipulated by the rationally designed RAFT agent at 75°C in THF for a preset time. The number-average molecular weight of the amphiphilic copolymer determined by conventional GPC was 6435 Da and the polydispersity index (PDI) was 1.22. The GPC traces of PEG-b-PSPMA and macro-CTA precursor are shown in Fig. 2. It can be seen that the molecular weight of PEG-PSPMA shifts toward the highmolecular weight region without any trace of the macro RAFT agent. This shows that PEG-CTA is an efficient, macro, chain transfer agent for the polymerization of SPMA with low polydispersity. The content of SP units was calculated by following equation:
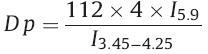

|
Download:
|
| Scheme 1.Synthesis of diblock copolymer PEG-b-PSPMA (a) and photo-induced isomerization between SP and MC (b). | |
|
Download:
|
| Fig. 1.1H NMR spectra of (a) SPMA in CDCl3, (b) PEG-CTA in DMSO-d6 and (c) PEG-b-PSPMA in DMSO-d6 at room temperature. | |

|
Download:
|
| Fig. 2.GPC curves of PEG-b-PSPMA and the corresponding precursor PEG-CTA. | |
The Dps of SP units in copolymers polymerized 24 h and 48 h were 9 and 18, respectively. The results obtained by 1H NMR are in good agreement with the value estimated by GPC, and suggested that the polymerization of SPMA proceeds in a well-controlled manner. However, The PDIs of the photo-responsive copolymers ranged between 1.22 and 1.52, which are somewhat higher than those reported for the controlled polymerization of styrene and methacrylate monomers [33,34], but lower compared to those spiropyran-based copolymers synthesized by conventional free radical polymerization [35,36]. The slightly higher molecular weight distributions of the copolymers are attributed to the low molecular weights of the polymers and the polymerization of highly functional monomers (SPMA) [37] and are similar to those reported in the literature for P4VP-co-PSPMA copolymer analogs synthesized by RAFT [10] and PMMA-co-PSPMA by ATRP [38].
Owing to the light-switchable hydrophobic PSPMA segment and hydrophilic PEG segment, the PEG112-b-PSPMA9 copolymer can self-assemble into micelles in water. The critical micellization concentration (CMC) of the PEG112-b-PSPMA9 determined by fluorescence spectrometer using pyrene as fluorescent probe was 0.01 mg mL-1. TEM and DLS measurements were performed to determine the morphology and size of the self-assemblies. Fig. 3A shows the dimension of assembles measured by DLS. The micelles have a number-average hydrodynamic diameter (Dh) of 157.3 nm and a polydispersity of 0.188. The PDI of the micellar size was a little larger compared to other self-assembled micelles. However, this is a common phenomenon for spiropyran containing copolymers because of the dynamic equilibrium between SP and MC. As shown in Fig. 3B, the PEG112-b-PSPMA9 self-assembled into spherical micelles in water with a diameter of about 100 nm.

|
Download:
|
| Fig. 3.Characterizations of PEG112-b-PSPMA9 micelles: (A) DLS size distribution and (B) TEM image. | |
Due to the light-responsive SP groups in the copolymer, the micellar morphology was enabled to be light-switchable because of the shift of hydrophobic–hydrophilic balance. It is well known that the SP moieties display two stable states, which could be reversibly inter-converted upon irradiation with UV and visible light. Generally, the absorption spectrum of the SP form in aqueous solution does not show any absorption band larger than 400 nm, whereas the MC form displays a characteristic absorption band at about 560 nm [39]. Therefore, the reversible inter-conversion of the SPMA units incorporated in the polymer chains can be studied via their UV/vis absorption spectra. First, 365 nm UV light was employed to irradiate the micelles solution (0.1 mg mL-1) at different times. As shown in Fig. 4A, the micellar solution transformed from colorless to purple. At the same time, the peak at 565 nm was gradually enhanced with increasing UV irradiation time, indicating that the closed, colorless SP form isomerized into the colored, opened MC form. Moreover, it can be found in Fig. 4A that the two curves almost coincide with each other after irradiating for 6 min and 7 min, demonstrating that the solution had reached a light-stationary state after 6 min of UV irradiation. Then, the ‘‘excited’’ micellar solution was exposed to 550 nm visible light for 70 min. The UV/vis absorption spectra were recorded at pre-arranged time intervals. In this process, the solution converted back to colorless with a decreasing of the absorption peak at 565 nm as shown in Fig. 4B. These aforementioned results indicated the reversible conversion between SP and MC. The t1/2 of the MC back to SP process is 13.5 min and 18.3 min for polymer concentration 0.1 mg mL-1 and 0.5 mg mL-1, respectively. Increasing the SP moiety immobilized in polymer chain induced just a little increase in isomerization kinetic (t1/2 = 15.2 min for PEG112-b-PSPMA18 with a concentration 0.1 mg mL-1). To test the cyclic reversible response ability, the sample was alternatively irradiated with 365 nm UV light and 550 nm visible light. The change of MC absorbance, monitored by UV spectroscopy is shown in Fig. 5. The reversible isomerization can take place very well in the beginning several cycles. However,there was a decline of the reversibility with the irradiation cycles, which was caused by an inevitable degradation of the dyes [40].
DLS and TEM measurements were also conducted to monitor the hydrophobic–hydrophilic balance shifting process. Fig. 6 shows the diameter change of the micelles after irradiated by UV/vis light at different times. The results show that the average diameter of assembles decreased significantly after irradiating with UV light. Adjusting the light to 550 nm visible light for 50 min and the diameter gradually increased. TEM was used to further confirm the evolution of the assemble dimension. The TEM images in Fig. 7 displayed, after irradiation for 7 min under UV light, that most of the sphere micelles dissociated, but a few smaller micelles with diameters of about 40 nm still existed. Since the isomerization is a dynamic equilibrium, the SP moiety cannot totally switch to MC moiety, therefore, the spherical micelles were not totally dissociated after UV irradiating. When subsequently irradiated the micelles with visible light for 50 min, the diameter increased to about 100 nm, which is comparable to that of before irradiation. However, it is noteworthy that these re-assembled micelle sizes are not uniform and some aggregates existed. The H-stack between planar MC molecules, whose p-bonding network extends over the entire molecular framework, is a plausible reason for this phenomenon [41]. These results indicate that the diameter of the micelles can be reversibly regulated by light.

|
Download:
|
| Fig. 4.Photo-isomerization process of PEG112-b-PSPMA9 micelles (0.1 mg mL-1) after irradiating with different lights tracked by UV–vis spectroscopy: (A) 365 nmUV light for 7 min (B) subsequent 550 nm visible light for 70 min. | |

|
Download:
|
| Fig. 5.Reversible photo-switching process of PEG112-b-PSPMA9 micelles (0.1 mg mL-1) alternatively irradiating with UV light (6 min) and vis light (70 min) for several cycles. | |

|
Download:
|
| Fig. 6.Size distributions of PEG112-b-PSPMA9 micelles after irradiation with UV/visible light for different times (A) and size evolvement after irradiation with UV/visible light for different times (B). | |

|
Download:
|
| Fig. 7.TEM images of PEG112-b-PSPMA9 micelles after irradiation with: (A) 365 nm UV light irradiation for 7 min and (B) subsequent 550 nm visible light irradiation for 50 min. | |
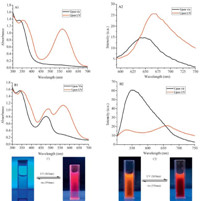
Fluorescence resonance energy transfer (FRET) is a nonirradiation energy transfer process between light-absorbing donors and light-receiving acceptors occurring in nano-scale. It is required that the emission band of the donors should overlap significantly with the absorption bands of the acceptor and the distance between them is within the Fo¨ rster radius (generally 1–10 nm) [42]. As we mentioned above, the spiropyran molecules have two stable states: SP form and MC form. The SP form exhibits no fluorescence emission, but the MC form has a strong emission between 600 nm to 700 nm. The nitrobenzoxadiazolyl derivative dyes are a good ‘‘donor’’ to MC [43,44]. When the NBD dye was loaded in the PEG112-b-PSPMA9 copolymer micelles, the micelles containing both the donor (NBD dye) and acceptor (spiropyran) may serve as the scaffold for the FRET process. Fig. 8A shows the absorption and fluorescence spectra of the aqueous micellar solution upon visible (550 nm) and UV (365 nm) light irradiation. Upon visible light, SP moieties in the micelles exhibited no absorption from 450 nm to 700 nm, whereas under UV irradiation, a new absorption band at ca. 565 nm appeared corresponding to the MC form (Fig. 8A1). Under irradiation at λex = 570 nm, the spiropyran molecules in MC form emitted strong fluorescence as shown in Fig. 8A2. The absorption spectrum and fluorescence intensity of the NBD in the micelle systems upon visible and UV light irradiation are illustrated in Fig. 8B. Upon visible light, spiropyran moieties in the SP form exhibited no absorption from 500 nm to 700 nm, with only an absorbance band of NBD at 460 nm observed. Otherwise, there was only one emission peak of NBD at 550 nm in the fluorescence spectra. Upon UV (365 nm) irradiation, the absorbance of MC at about 560 nm was observed. And a new emission band at 675 nm, corresponding to MC form, appeared in the fluorescence spectra after 3 min of UV irradiation. The fluorescence color of the NBD-loaded micelle solution changed to red from orange (Fig. 8C). However, the characteristic fluorescence emission intensity of NBD decreased significantly compared to that before UV irradiation. This phenomenon is ascribed to the quenching of NBD fluorescence by the MC during the FRET process. Otherwise, there was a 20 nm blue-shift of the NBD characteristic fluorescence emission during this process.
Moreover, the fluorescence of the complex micellar solution can be reversibly quenched and recovered by alternating irradiation between the visible and UV light. The color of the micellar solution was reversibly changed correspondingly (Fig. 8C). From the perspective of an energy-level match, the energy of the firstexcited singlet state of NBD was estimated to be 2.50 eV based on its absorption and emission maxima. Similar calculations gave a first-excited singlet-state energy of 3.65 eV for the SP form and 2.05 eV for the MC form of the spiropyran moiety [44]. Therefore, before UV irradiation, no fluorescence quenching of NBD was observed. However, upon UV irradiation, the SP converted into MC and then the energy transfer from NBD to the spiropyran became efficient. These effects validate the energy transfer from NBD to SP is impossible, but is possible to the MC.
Lastly, the reversibility of fluorescence modulation of NBD loaded micelles via alternatively cycled irradiation with UV and visible light was studied. The light triggered cycle of reversible fluorescence ‘‘turn on’’ and ‘‘turn off’’ was monitored by fluorescence spectrometer based on the emission of NBD at 530 nm. The reversible behavior of the complex micelles performed well even after several irradiation cycles (Fig. 9). However, with repeated irradiation cycles, the fluorescence intensity of the ‘‘off state’’ (upon UV irradiation) increased, while the fluorescence intensity of the ‘‘on state’’ (upon vis irradiation) decreased, because of the irreversible photodamage of some NBD molecules and spiropyran reversibly fluorescence ‘‘turn on’’ and ‘‘turn off’’ was monitored by fluorescence spectrometer based on the emission of NBD at 530 nm. The reversible behavior of the complex micelles displayed well even after several irradiation cycles (Fig. 9). However, with repeat irradiation cycles, the fluorescence intensity of the ‘‘off state’’ (upon UV irradiation) increased, while the fluorescence intensity of moieties under repeated UV irradiation [43–45].
|
Download:
|
| Fig. 8.(A) Absorption and fluorescence spectra (λex = 570 nm) of PEG112-b-PSPMA9micelles dispersion without NBD. (B) Absorption and fluorescence spectra (λex = 492 nm) of NBD-loaded micelle dispersion upon UV- and vis-light irradiation. (C) The fluorescence color changes of the dispersions with (C2) and without (C1) NBD dye upon UV and vis irradiation. | |

|
Download:
|
| Fig. 9.TEM images of PEG112-b-PSPMA9 micelles after irradiation with: (A) 365 nm UV light irradiation for 7 min and (B) subsequent 550 nm visible light irradiation for 50 min. | |
Amphiphilic copolymers have been successfully prepared by one-step RAFT polymerization of SPMA, using PEG-CTA as the macro-RAFT agent. The self-assembling of the copolymer in water formed spherical micelles with PSPMA segment in the core and PEG segment as the shell. The diameter of the micelles has a reversible, light-switchable behavior due to the shift of hydrophobic– hydrophilic balance induced by the isomerization between the SP form and MC form. As a well matched ‘‘donor’’ to MC in the FRET process, NBD dyes encapsulated into the core of the micelles formed the light-responsive fluorescence emission system. Upon UV irradiation, the latent fluorescence moiety converts to the MC form, and then the FRET process was turn-on. But when the light changed to visible, concomitant with the MC isomerizing back to SP, the FRET process was reversibly turned off. The photoswitching fluorescence between orange and red can be facilely achieved and repeated over many cycles by alternative UV/vis irradiation. These photochromic properties and the photo-switchable FRET process of this amphiphilic copolymer enable the potential application in drug delivery and cell imaging and tracking synchronously.
This work was financially supported by the National Natural Science Foundation of China (Nos. 51073106 and 51121001) and Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (No. IRT1026). The Analytical and Testing Center of Sichuan University provided TEM analysis.
| [1] | Y.L. Yu, M. Nakano, T.Ikeda, Photomechanics: directed bending of a polymer film by light, Nature 425 (2003) 145. |
| [2] | H. Lee, W. Wu, J.K. Oh, et al., Light-induced reversible formation of polymeric micelles, Angew. Chem. Int. Ed.46 (2007) 2453-2457. |
| [3] | V.K. Kotharangannagari, A. Sànchez-Ferrer, J. Ruokolainen, R. Mezzenga, Photoresponsive reversible aggregation and dissolution of rod-coil polypeptide diblock copolymers, Macromolecules 44 (2011) 4569-4573. |
| [4] | S. Dai, P. Ravi, K.C.Tam, pH-responsive polymers: synthesis, properties and applications, Soft Matter 4 (2008) 435-449. |
| [5] | S. Yusa, M. Sugahara, T. Endo, Y.Morishima, Preparation and characterization of a pH-responsive nanogel based on a photo-cross-linked micelle formed from block copolymers with controlled structure, Langmuir 25 (2009) 5258-5265. |
| [6] | G. Wu, S.C. Chen, Q. Zhan, Y.Z. Wang, Well-defined amphiphilic biodegradable comb-like graft copolymers: their unique architecture-determined LCST and UCST thermoresponsivity, Macromolecules 44 (2011) 999-1008. |
| [7] | A.P. Vogt, B.S. Sumerlin, Temperature and redox responsive hydrogels from ABA triblock copolymers prepared by RAFT polymerization, Soft Matter 5 (2009) 2347-2351. |
| [8] | J.M. Hu, G.Y. Zhang, Y.H. Geng, S.Y.Liu, Micellar nanoparticles of coil-rod-coil triblock copolymers for highly sensitive and ratiometric fluorescent detection of fluoride Ions, Macromolecules 44 (2011) 8207-8214. |
| [9] | C.J. Chen, Q. Jin, G.Y. Liu, et al. Reversibly light-responsive micelles constructed via a simple modification of hyperbranched polymers with chromophores, Polymer 53 (2012) 3695-3703. |
| [10] | C.Q. Huang, Y. Wang, C.Y. Hong, C.Y. Pan, Spiropyran-based polymeric vesicles: preparation and photochromic properties, . Macromol. Rapid Commun 32 (2011) 1174-1179. |
| [11] | C.S. Brazel, Magnetothermally-responsive nanomaterials: combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release, Pharm. Res. 26 (2009) 644-656. |
| [12] | M. Irie, Photoresponsive polymers.Reversible bending of rod-shaped acrylamide gels in an electric field, Macromolecules 19 (1986) 2890-2892. |
| [13] | H.T.T. Duong, C.P. Marquis, M. Whittaker, T.P. Davis, C.Boyer, Acid degradable and biocompatible polymeric nanoparticles for the potential codelivery of therapeutic agents, Macromolecules 44 (2011) 8008-8019. |
| [14] | Y.H. Wang, M. Zheng, F.H. Meng, et al.Branched polyethylenimine derivatives with reductively cleavable periphery for safe and efficient in vitro gene transfer, Biomacromolecules 12 (2011) 1032-1040. |
| [15] | T.A. Darwish, R.A. Evans, M. James, et al., CO2 triggering and controlling orthogonally multiresponsive photochromic systems, J. Am. Chem. Soc.132 (2010) 10748-10755. |
| [16] | D.H. Han, X. Tong, O. Boissière, Y. Zhao, General strategy for making CO2-switchable polymers, ACS Macro Lett.1 (2012) 57-61. |
| [17] | J.P. Magnusson, A. Khan, G. Pasparakis, et al., Ion-sensitive "isothermal" responsive polymers prepared in water, J. Am. Chem. Soc.130 (2008) 10852-10853. |
| [18] | D.B. Liu, W.W. Chen, K. Sun, et al., Resettable, multi-readout logic gates based on controllably reversible aggregation of gold nanoparticles, Angew. Chem. Int. Ed. 50 (2011) 4103-4107. |
| [19] | M. Irie, T. Fukaminato, T. Sasaki, N. Tamai, T.Kawai, Organic chemistry: a digital fluorescent molecular photoswitch, Nature 420 (2002) 759-760. |
| [20] | Y.M. Li, Y.F. Qian, T. Liu, G.Y. Zhang, S.Y.Liu, Light-triggered concomitant enhancement of magnetic resonance imaging contrast performance and drug release rate of functionalized amphiphilic diblock copolymer micelles, Biomacromolecules 13 (2012) 3877-3886. |
| [21] | S. Wang, Y. Song, L.J. Jiang, Photoresponsive surfaces with controllable wettability, Photochem. Photobiol. C 8 (2007) 18-29. |
| [22] | M.Q. Zhu, G.F. Zhang, C. Li, et al., Reversible two-photon photoswitching and twophoton imaging of immunofunctionalized nanoparticles targeted to cancer cells, J. Am. Chem. Soc. 133 (2011) 365-372. |
| [23] | A. Nayak, H. Liu, G. Belfort, An optically reversible switching membrane surface, Angew. Chem. Int. Ed. 45 (2006) 4094-4098. |
| [24] | Y.Zhao, Light-responsive block copolymer micelles, Macromolecules 45 (2012) 3647-3657. |
| [25] | X.K. Liu, M. Jiang, Optical switching of self-assembly: micellization and micellehollow-sphere transition of hydrogen-bonded polymers, Angew. Chem. Int. Ed. 45 (2006) 3846-3850. |
| [26] | L. Ming, L.Y. Gu, Q. Zhang, M.Z. Xue, Y.G. Liu, Preparation and study of photoswitchable fluorescence nanoparticles based on spirobenzopyran, Chin. Chem. Lett. 24 (2013) 1014-1018. |
| [27] | J.L. Mynar, A.P. Goodwin, J.A. Cohen, et al., Two-photon degradable supramolecular assemblies of linear-dendritic copolymers, Chem. Commun. 20 (2007) 2081-2082. |
| [28] | D.H. Han, X. Tong, Y.Zhao, Fast photodegradable block copolymer micelles for burst release, Macromolecules 44 (2011) 437-439. |
| [29] | Y. Zhao, J. Bertrand, X. Tong, Y.Zhao, Photo-cross-linkable polymer micelles in hydrogen-bonding-built layer-by-layer films, Langmuir 25 (2009) 13151-13157. |
| [30] | J.T. Lai, D. Filla, R.Shea, Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents, Macromolecules 35 (2002) 6754-6756. |
| [31] | D.J. Chung, Y. Ito, Y. Imanishi, Preparation of porous membranes grafted with poly (spiropyran-containing methacrylate) and photocontrol of permeability, J. Appl. Polym. Sci. 51 (1994) 2027-2033. |
| [32] | S.I. Yusa, Y. Yokoyama, Y.Morishima, Synthesis of oppositely charged block copolymers of poly(ethylene glycol) via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water, Macromolecules 42 (2009) 376-383. |
| [33] | Y.K. Chong, J. Krstina, T.P.T. Le, et al., Thiocarbonylthio compounds [SC(Ph)S-R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization).ole of the free-radical leaving group (R), R Macromolecules 36 (2003) 2256-2272. |
| [34] | Y. Cao, X.X. Zhu, J.T. Luo, H.Y.Liu, Effects of substitution groups on the RAFT polymerization of N-alkylacrylamides in the preparation of thermosensitive block copolymers, Macromolecules 40 (2007) 6481-6488. |
| [35] | K. Sumaru, M. Kameda, T. Kanamori, T.Shinbo, Characteristic phase transition of aqueous solution of poly (N-isopropylacrylamide) functionalized with spirobenzopyran, Macromolecules 37 (2004) 4949-4955. |
| [36] | A.Y. Bobrovsky, N.I. Boiko, V.P. Shibaev, Photosensitive cholesteric copolymers with spiropyran-containing side groups: novel materials for optical data recording, Adv. Mater. 11 (1999) 1025-1028. |
| [37] | D.S. Achilleos, M. Vamvakaki, Multiresponsive spiropyran-based copolymers synthesized by atom transfer radical polymerization, Macromolecules 43 (2010) 7073-7081. |
| [38] | M. Piech, N.S.Bell, Controlled synthesis of photochromic polymer brushes by atom transfer radical polymerization, Macromolecules 39 (2006) 915-922. |
| [39] | G.Y. Jiang, Y.L. Song, X.F. Guo, D.Q. Zhang, D.B. Zhu, Organic functional molecules towards information processing and high-density information storage, Adv. Mater. 20 (2008) 2888-2898. |
| [40] | M.Q. Zhu, L.Y. Zhu, J.J. Han, et al., Spiropyran-based photochromic polymer nanoparticles with optically switchable luminescence, J. Am. Chem. Soc. 128 (2006) 4303-4309. |
| [41] | D.S. Achilleos, T.A. Hatton, M. Vamvakaki, Light-regulated supramolecular engineering of polymeric nanocapsules, J. Am. Chem. Soc. 134 (2012) 5726-5729. |
| [42] | K.E. Sapsford, L. Berti, I.L. Medintz, Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations, Angew. Chem. Int. Ed. 45 (2006) 4562-4589. |
| [43] | Y. Wang, C.Y. Hong, C.Y.Pan, Spiropyran-based hyperbranched star copolymer: synthesis, phototropy, FRET, and bioapplication, Biomacromolecules 13 (2012) 2585-2593. |
| [44] | J. Chen, F. Zeng, S.Z. Wu, Q.M. Chen, Z. Tong, A core-shell nanoparticle approach to photoreversible fluorescence modulation of a hydrophobic dye in aqueous media, Chem. Eur. J. 14 (2008) 4851-4860. |
| [45] | J. Föling, S. Polyakova, V. Belov, et al.Synthesis and characterization of photoswitchable fluorescent silica nanoparticles, Small 4 (2008) 134-142. |




