The principles of green chemistry have been introduced to eliminate or reduce the use or generation of hazardous materials in chemical processes. One of the key areas of green chemistry is the replacement of hazardous solvents with environmentally benign ones or the elimination of solvents altogether [1, 2, 3]. One strategy is the use of ionic liquids (ILs) that are defined as salts in liquid form at or below 100℃. ILs have gained much attention as ‘‘designer solvents and/or catalysts’’ for a diversity of chemical applications due to their special properties such as high polarity,good solvating capability,wide liquid range,high thermal stability,negligible vapour pressure,great selectivity,ease of isolation and reusability [4, 5, 6]. Among the different kinds of ILs,Brønsted acidic ionic liquids,which have a functional group such as -SO3H in their framework,have been designed as green substitute for solid acids and traditional mineral liquid acids such as sulfuric acid and hydrochloric acid in chemical processes [7, 8, 9, 10, 11].
In recent years,considerable efforts from both academic and industrial researchers have been focused especially on the design and development of multi-component reactions (MCRs) for the generation of libraries of heterocyclic compounds [12].
There has been a renewed and growing interest in MCRs as notable synthetic tools for the synthesis of structurally diverse complex molecules,such as novel biologically and pharmaceutically active compounds. MCRs offer several attractive features to the chemists,including access to a large number of novel and diverse structures,low production and environmental costs due to high convergence,atom economy,high selectivity and simple purification [13, 14, 15, 16].
Xanthenes and their derivatives have received special attention due to their wide range of biological and pharmaceutical activities such as antibacterial [17],antiviral [18] and antitumor effects [19]. In addition,they can be employed as dyes [20],pH sensitive fluorescent materials for visualization of biomolecules [21] and utilized in laser technology [22]. Thus,the synthesis of xanthene derivatives currently is of much importance.
Various approaches have been reported for the synthesis of xanthene derivatives in the literature [23, 24, 25, 26]. One of the most simple and general methods for the synthesis of this type of compounds involves a one-pot MCR of aldehydes (1 equiv.) with bnaphthol (1 or 2 equiv.) and dimedone (1 or 2 equiv.),in the presence of an acidic or other type of catalysts. A variety of catalysts have been applied for this reaction,such as ferric hydrogen sulfate [27],proline triflate [28],NaHSO4·SiO2 [29], strontium triflate [30],Zr(HSO4)4 [31],RuCl4 [32],P2O5/Al2O3 [33], BF3·SiO2 [34],oxalic acid [35],(DBH)/kaolin [36],ZnO nanoparticles [37],molecular iodine [38, 39],heteropoly acid [40, 41], silica sulfuric acid [42, 43],amberlyst-15 [44],nano-TiO2 [45],KAl(SO4)2·12H2O (alum) [46],NaHSO4/ionic liquid ([bmim]BF4) [47],montmorillonite K-10 [48],p-TSA [49],cyanuric chloride [50], H4[SiW12O40] [51],Yb(OTf)3 [52] and SuSA [53]. However,many of these procedures suffer from one or more disadvantages including the use of toxic metals and volatile organic solvents,low yields, long reaction times,tedious work-up procedures,expensive reagents,high catalyst loading,high temperature and hash reaction conditions.
To avoid these drawbacks and develop useful synthetic methodologies,herein,we wish to report a simple,green and efficient method for the synthesis of xanthene derivatives using DSIMHS as an eco-friendly catalyst with high catalytic activity under solvent-free conditions. To the best of our knowledge,this methodology has not been reported in the literature. 2. Experimental
All chemicals were purchased from Merck or Fluka Chemical Companies. All yields refer to the isolated products. Products were characterized by their physical constants and comparison with authentic samples. Progress of the reactions was monitored by thin layer chromatography (TLC) analyses using silica gel SIL G/UV 254 plates.
Melting points were recorded on an electrothermal digital melting point apparatus model IA9100 in open capillary tubes. IR spectra were recorded on a Perkin-Elmer model Spectrum One FTIR Spectrometer. The 1H NMR (300 or 400 MHz) was run on a Bruker Avance DPX-250 FT-NMR spectrometer (δ in ppm).
Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes: A mixture of β-naphthol (2 mmol),aldehyde (1 mmol) and DSIMHS (0.25 mmol) was stirred and heated in an oil-bath at 90℃ for an appropriate period of time. The progress of the reaction was followed by TLC analyses. After the completion of the reaction,the reaction mixture was cooled to room temperature,10 mL of H2O was added,stirred for 5 min and filtered to remove the catalyst. DSIMHS is soluble in water and the product precipitated with high purity. Then,2 mL of hot EtOH was added to the resulting solid product,stirred for 5 min,and filtered. Finally,the solid residue was recrystallized from EtOH to give the pure product.
Synthesis of 12-aryl-tetrahydrobenzo[a]xanthene-11-ones: A mixture of β-naphthol (1 mmol),dimedone (1 mmol),aldehyde (1 mmol) and DSIMHS (0.25 mmol) was stirred and heated in an oil-bath at 55℃ for an appropriate period of time. The reaction was monitored by TLC analyses. After the completion of the reaction, the reaction mixture was cooled to room temperature,10 mL of H2O was added,stirred for 5 min and filtered to remove the catalyst. Then,2 mL of hot EtOH was added to the resulting solid product,stirred for 5 min,and filtered. Finally,the solid residue was recrystallized from EtOH to give the pure product.
Synthesis of 1,8-dioxo-octahydroxanthenes: A mixture of dimedone (2mmol),aldehyde (1mmol) and DSIMHS (0.25mmol) was stirred and heated in an oil-bath at 55℃ for an appropriate period of time. After the completion of the reaction,asmonitored by TLC analyses,the reaction mixture was cooled to roomtemperature, 10 mL of H2O was added,stirred for 5 min and filtered to remove the catalyst. Then,2 mL of hot EtOH was added to the resulting solid product,stirred for 5 min,and filtered. Finally,the solid residuewas recrystallized from EtOH to give the pure product. Spectroscopic data for the selected products are as follows:
14-(Phenyl)-14H-dibenzo[a,j]xanthene (Table 2,entry 1): IR (KBr,cm-1): ν 3070,3020,1620,1590,1430,1400,1250,1150, 1075,825,740; 1H NMR (300 MHz,CDCl3): δ 6.46 (s,1H),6.96 (t, 1H,J = 7.2 Hz),7.12 (t,2H,J = 7.2 Hz),7.36-7.58 (m,8H),7.74-7.81 (m,4H),8.37 (d,2H,J = 8.4 Hz).
14-(4-Methylphenyl)-14H-dibenzo[a,j]xanthene (Table 2,entry 9): IR (KBr,cm-1): ν 3068,3022,1620,1590,1512,1395,1248,1110,810,740; 1H NMR (CDCl3,300 MHz): δ 2.11 (s,3H),6.43 (s, 1H),6.93 (d,2H,J = 7.8 Hz),7.22-7.36 (m,8H),7.42-7.81 (m,4H), 8.37 (d,2H,J = 8.4 Hz).
12-(4-Isoprpylphenyl)-9,9-dimethyl-8,9,10,12 tetrahydrobenzo[ a]xanthen-11-one(Table 4,entry 12): IR (KBr,cm-1): ν 3050, 2950,2900,2870,1642,1618,1590,1504,1460,1368,1220,1140, 1020,830,815,740; 1H NMR (CDCl3,400 MHz): δ 1.01 (s,3H),1.14 (s,6H),1.16 (s,3H),2.23 and 2.30 (AB system,2H,J = 30 Hz),2.48- 2.59 (d,2H,J = 45 Hz),2.77 (t,1H,J = 6.8 Hz),5.70 (s,1H),7.03 (d, 1H,J = 7.2 Hz),7.19-7.46(m,5H),7.78 (t,3H,J = 10 Hz),8.05 (d,1H, J = 8 Hz).
12-(4-Cyanophenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[ a]xanthen-11-one (Table 4,entry 13): IR (KBr,cm-1): ν 3070, 2956,2931,2869,2220,1651,1618,1594,1515,1460,1370,1220, 1175,1140,1020,838,802,740; 1H NMR (CDCl3,400 MHz): δ 0.97 (s,3H),1.15 (s,3H),2.25 and 2.37 (AB system,2H,J = 16 Hz),2.61 (s,2H),5.78 (s,1H),7.37 (d,1H,J = 8.8 Hz),7.41-7.49 (m,6H),7.84 (t,3H,J = 8.4 Hz).
9-(2-Mehtoxyphenyl)-3,3,6,6-tetramethyl-1,8-dioxo-octahydroxanthene (Table 6,entry 12): IR (KBr,cm-1): ν 3010,2950, 2870,1650,1620,1590,1490,1460,1360,1250,1020,1200,1000, 1160,1140,1118,750; 1H NMR (CDCl3,400 MHz): δ 0.97 (s,6H), 1.11 (s,6H),2.14 and 2.23 (d,2H,J = 6.4 Hz),2.39 and 2.48 (d,2H, J = 17.4 Hz),3.79 (s,3H),4.87 (s,1H),6.77 (d,1H,J = 8 Hz),6.89 (dt, 1H,J = 7.4 Hz,J = 1.2 Hz),7.12 (m,1H),7.43 (dd,1H,J = 7.4 Hz,J 1.6 Hz).
9-(4-Cyanophenyl)-3,3,6,6-tetramethyl-1,8-dioxo-octahydroxanthene (Table 6,entry 13): IR (KBr,cm-1): ν 3050,2950,2870, 2210,1660,1620,1600,1500,1460,1360,1200,1160,1140,1105, 850; 1H NMR(CDCl3,400 MHz): δ 0.99 (s,6H),1.13 (s,6H),2.17 and 2.26 (d,2H,J = 16.4 Hz),2.5 (d,2H,J = 1.2 Hz),4.78 (s,1H),7.43 (dd, 2H,J = 9.6 Hz,J = 1.6 Hz),7.54 (dd,2H,J = 6.6 Hz,J = 1.6 Hz). 3. Results and discussion
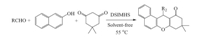
In continuation of our ongoing research program on the development of new applications of DSIMHS in organic synthesis [54, 55],we decided to study the preparation of 14-aryl-14Hdibenzo[ a,j]xanthenes from aldehydes and β-naphthol in the presence of this catalyst (Scheme 1). For this purpose,the condensation of β-naphthol (2 mmol) with benzaldehyde (1 mmol) was selected as a model reaction and the optimization of the reaction conditions was performed using various amounts of DSIMHS at different temperatures under solvent-free conditions. The results are summarized in Table 1.

|
Download:
|
| Scheme 1.Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes in the presence of DSIMHS under solvent-free conditions. | |
| Table 1 The effect of different amounts of DSIMHS and temperature on the synthesis of 14-(phenyl)-14H-dibenzo[a,j]xanthene.a |
As it shown in Table 1,the best result was obtained by carrying out the reaction using 0.25 mmol of DSIMHS at 90℃ (Table 1, entry 5). Increasing the amount of the catalyst and the temperature of the reaction did not improve the yields and reaction times. In addition,results indicated that when the reaction proceeded at room temperature in 240 min,the yield of the corresponding product was low (Table 1,entry 1). The solvent-free reaction was also tested at 90℃ in the absence of the catalyst and no significant amount of product was observed after long reaction time (Table 1, entry 2).
In order to study the generality of this procedure,a variety of aromatic aldehydes were applied under the optimal reaction conditions. The results are shown in Table 2. The nature of the functional group on the aromatic ring of the aldehyde exerted a strong influence on the reaction time. It could be concluded that the aldehydes substituted with electron-withdrawing groups reacted very well and produced the corresponding xanthenes in good to excellent yields in shorter times than aldehydes with electron-donating groups.
| Table 2 DSIMHS catalyzed synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes.a |
After the successful synthesis of a series of 14-aryl-14Hdibenzo[ a,j]xanthenes,we turned our attention toward the synthesis of 12-aryl-tetrahydrobenzo[a]xanthene-11-ones,in the presence of DSIMHS (Scheme 2). In order to determine the optimal conditions,we investigated the MCR of β-naphthol (1 mmol) with dimedone (1 mmol) and benzaldehyde (1 mmol) in the presence of various amounts of the catalyst,at different temperatures ranging from 25℃ to 65℃ in the absence of solvent (Table 3).
|
Download:
|
| Scheme 2.Three-component synthesis of 12-aryl-tetrahydrobenzo[a]xanthene-11-ones in the presence of DSIMHS under solvent-free conditions. | |
| Table 3 The effect of different amounts of DSIMHS and temperature on the synthesis of 12- phenyl-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one.a |
According to Table 3,we found that 0.25 mmol of DSIMHS was adequate to accomplish the reaction effectively at 55℃ (Table 3,entry 4). Using smaller amounts of the catalyst resulted in lower yields,while higher amounts of the catalyst did not affect the reaction times and yields and in the absence of the catalyst,no appreciable product could be detected. Moreover, when the same reaction was performed at 25℃,the corresponding product was obtained in very low yield after prolonged reaction time.
To generalize the suitability of this protocol,the reaction was extended to different substituted aromatic aldehydes to prepare a series of 12-aryl-tetrahydrobenzo[a]xanthene-11-ones. In all cases,aldehydes bearing electron-donating groups needed slightly longer time to complete the reaction as compared to aldehydes containing halogens or other electron-withdrawing groups. The results are summarized in Table 4.
| Table 4 DSIMHS catalyzed synthesis of 12-aryl-tetrahydrobenzo[a]xanthene-11-ones.a |
Finally,we evaluated the catalytic activity of DSIMHS in the synthesis of 1,8-dioxo-octahydroxanthenes. In this regard,the reaction between dimedone (2 mmol) and benzaldehyde (1 mmol) was chosen as a model reaction using different amounts of the catalyst,at various temperatures under solvent-free conditions (Scheme 3). As can be seen from Table 5,the shortest time and best yield was achieved using 0.25 mmol of the reagent at 55℃. It is interesting to note that the yield of the reaction was not significantly affected by increasing the amount of the catalyst and temperature. Also,in the absence of the catalyst or at room temperature,only a trace amount of the product was obtained indicating that the catalyst and elevated temperature are necessary for the reaction.

|
Download:
|
| Scheme 3.Ionic liquid catalyzed synthesis of 1,8-dioxo-octahydroxanthenes in the presence of DSIMHS under solvent-free conditions. | |
| Table 5 The effect of different amounts of DSIMHS and temperature on the synthesis of 9-(phenyl)-3,3,6,6-tetramethyl-1,8-dioxo-octahydroxanthenes.a |
After the optimization of the reaction,we extended the condensation of dimedone with various aromatic aldehydes to further explore the scope and limitations of this reaction. The results showed that the reaction proceeds very efficiently and in all cases,aromatic aldehydes substituted with either electrondonating or electron-withdrawing groups underwent the reaction successfully and gave the desired products in good to excellent yields (Table 6).
| Table 6 DSIMHS catalyzed synthesis of 1,8-dioxo-octahydroxanthenes.a |
In Scheme 4,a plausible mechanism has been offered for the synthesis of xanthene derivatives in the presence of DSIMHS under solvent-free conditions. On the basis of this mechanism,DSIMHS donates the proton to the oxygen atom of the aldehyde and activates it. Then,nucleophilic β-naphthol (A) or dimedone (B) attacks the carbonyl group of the activated aldehyde and by removing H2O,the Knoevenagel products (I or II) is generated. The following addition of these intermediates to A or B,gives the acyclic adduct intermediate,which undergoes an intramolecular cyclization with the participation of two hydroxyl groups to afford the xanthene derivatives (Scheme 4).
|
Download:
|
| Scheme 4.Proposed mechanism for the synthesis of xanthene derivatives in the presence of DSIMHS under solvent-free conditions. | |
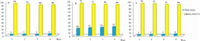
It is noteworthy to mention that the catalyst is recyclable and could be reused without significant loss of activity. Hence,we decided to examine the reusability and recycling performance of DSIMHS for the synthesis of xanthene derivatives. For this purpose, all the water added for filtering and washing the product was collected and washed with CH2Cl2 (2 × 10 mL) to remove organic impurities. Then,water was evaporated and the extracted catalyst was dried. It was found that the recovered catalyst could be reused directly in the next run without any loss of its activity even after the fourth run (Fig. 1).
|
Download:
|
| Fig. 1.Reusability of DSIMHS in the synthesis of (a) 14-(phenyl)-14H-dibenzo[a,j]xanthenes,(b) 12-phenyl-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one and (c) 9-(phenyl)-3,3,6,6-tetramethyl-1,8-dioxo-octahydroxanthenes. | |
As shown in Tables 7-9 the performance of DSIMHS was compared with the efficiency of other ILs reported in the literature. It is clear from these data that DSIMHS can act as an efficient and beneficial catalyst compared with the other mentioned reagents. For example,in Table 7,amount of the catalyst,reaction times and temperature in this work have been reduced relative to the other ILs. In addition,Table 9 shows that the reaction can be carried out using smaller amounts of DSIMHS in shorter reaction times compared to the other catalysts.
| Table 7 Comparison of the efficiency of various ILs in synthesis of 14-(phenyl)-14H-dibenzo[a,j]xanthene. |
| Table 8 Comparison of the efficiency of various ILs in synthesis of 12-(4-chlorophenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one. |
| Table 9 Comparison of the efficiency of various ILs in synthesis of 9-(4-bromophenyl)-3,3,6,6-tetramethyl-1,8-dioxo-octahydroxanthenes under solvent-free conditions. |
In conclusion,herein we described DSIMHS as an inexpensive, easily available,efficient,reusable and green catalyst for the synthesis of xanthenes in a simple one-pot protocol under solvent-free conditions with excellent yields. The obtained results show that the catalytic activity of DSIMHS is convincingly superior to other recently reported catalysts. In addition,other advantages of this methodology such as short reaction times,simplicity of operation and easy work-up,high reaction rates,lack of side reactions,ease of preparation and handling of the catalyst and simple experimental procedure makes it attractive for organic chemists. Further work to explore this novel catalyst in other organic transformations is in progress. Acknowledgment
The authors are thankful to the University of Guilan Research Council for the partial support of this work.
| [1] | P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, UK, 1998. |
| [2] | M. Poliakoff, M.J. Fitzpatric, T.R. Farren, P.T. Anastas, Green chemistry: science and politics of change, Science 297 (2002) 807-810. |
| [3] | I.T. Horváth, P.T. Anastas, Innovations and green chemistry, Chem. Rev. 107 (2007) 2169-2173. |
| [4] | T. Welton, Room-temperature ionic liquids. Solvents for synthesis and catalysis, Chem. Rev. 99 (1999) 2071-2084. |
| [5] | T. Welton, Ionic liquids in catalysis, Coord. Chem. Rev. 248 (2004) 2459- 2477. |
| [6] | P. Wasserscheid, W. Keim, Ionic liquids-new ""solutions"" for transition metal catalysis, Angew. Chem. Int. Ed. 39 (2000) 3772-3789. |
| [7] | M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, 3-Methyl-1-sulfonic acid imidazolium chloride as a new, efficient and recyclable catalyst and solvent for the preparation of N-sulfonyl imines at room temperature, J. Iran. Chem. Soc. 7 (2010) 646-651. |
| [8] | M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, Ionic liquid 3-methyl-1- sulfonic acid imidazolium chloride as a novel and highly efficient catalyst for the very rapid synthesis of bis(indolyl)methanes under solvent-free conditions, Org. Prep. Proced. Int. 42 (2010) 95-102. |
| [9] | A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, A. Zare, An efficient method for the nitration of phenols with nano2 in the presence of 3-methyl-1-sulfonic acid imidazolium, Sci. Iran. Trans. C: Chem. Chem. Eng. 17 (2010) 31-36. |
| [10] | M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Rapid synthesis of 1-amidoalkyl-2-naphthols over sulfonic acid functionalized imidazolium salts, Appl. Catal. A: Gen. 400 (2011) 70-81. |
| [11] | S.L. Schreiber, Target-oriented and diversity-oriented organic synthesis in drug discovery, Science 287 (2000) 1964-1969. |
| [12] | A. Domling, Recent advances in isocyanide-based multicomponent chemistry, Curr. Opin. Chem. Biol. 6 (2002) 306-313. |
| [13] | J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005. |
| [14] | V. Polshettiwar, R.S. Varma, Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation, Pure. Appl. Chem. 80 (2008) 777-790. |
| [15] | A. Hasaninejad, A. Zare, M. Shekouhy, Highly efficient synthesis of triazolo[1, 2- a]indazole-triones and novel spiro triazolo[1,2-a]indazole-tetraones under solvent- free conditions, Tetrahedron 67 (2011) 390-400. |
| [16] | S. Stavber, Recent advances in the application of selectfluorTM F-TEDA-BF4 as a versatile mediator or catalyst in organic synthesis, Molecules 16 (2011) 6432- 6464. |
| [17] | J.P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, et al., H4SiW12O40 catalyzed onepot synthesis of 12-aryl-8, 9,10,12-tetrahydrobenzo[a] xanthen-11-ones under solvent-free conditions, Eur. J. Med. Chem. 13 (1978) 67-71. |
| [18] | J.M. Jamison, K. Krabill, A. Hatwalkar, E. Jamison, C. Tsai, Potentiation of the antiviral activity of poly r(A-U) by xanthene dyes, Cell. Biol. Int. Rep. 14 (1990) 1075-1084. |
| [19] | G.W. Rewcastle, G.J. Atwell, L. Zhuang, B.C. Baguley, W.A. Denny, Potential antitumor agents. 61. Structure-activity relationships for in vivo colon 38 activity among disubstituted 9-oxo-9H-xanthene-4-acetic acids, J. Med. Chem. 34 (1991) 217-222. |
| [20] | A.T. Peters, M.J. Bide, Amino derivatives of 1,8-naphthalic anhydride and derived dyes for synthetic-polymer fibres, Dyes Pigm. 6 (1985) 349-375. |
| [21] | C.G. Knight, T. Stephens, Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles, Biochem. J. 258 (1989) 683-687. |
| [22] | M. Ahmad, T.A. King, D.K. Ko, B.H. Cha, J. Lee, Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases, J. Phys. D: Appl. Phys. 35 (2002) 1473-1476. |
| [23] | A. Bekaert, J. Andrieux, M. Plat, New total synthesis of bikaverin, Tetrahedron Lett. 33 (1992) 2805-2806. |
| [24] | D.W. Knight, P.B. Little, The first efficient method for the intramolecular trapping of benzynes by phenols: a new approach to xanthenes, J. Chem. Soc. Perkin Trans. 1 15 (2001) 1771-1777. |
| [25] | C.W. Kuo, J.M. Fang, Synthesis of xanthenes, indanes, and tetrahydronaphthalenes via intramolecular phenyl-carbonyl coupling reactions, Synth. Commun. 31 (2001) 877-892. |
| [26] | J.Q. Wang, R.G. Harvey, Synthesis of polycyclic xanthenes and furans via palladium- catalyzed cyclization of polycyclic aryltriflate esters, Tetrahedron 58 (2002) 5927-5931. |
| [27] | H.R. Shaterian, A. Hosseinian, M. Ghashang, Ferric hydrogen sulfate as an efficient heterogeneous catalyst for environmentally friendly greener synthesis of 1,8- dioxo-octahydroxanthenes, Turk. J. Chem. 33 (2009) 233-240. |
| [28] | J. Li, L. Lu, W. Su, A new strategy for the synthesis of benzoxanthenes catalyzed by proline triflate in water, Tetrahedron Lett. 51 (2010) 2434-2437. |
| [29] | B. Das, K. Laxminarayana, M. Krishnaiah, Y. Srinivas, An efficient and convenient protocol for the synthesis of novel 12-aryl- or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one derivatives, Synlett (2007) 3107-3112. |
| [30] | J. Li, W. Tang, L. Lu, W. Su, Strontium triflate catalyzed one-pot condensation of bnaphthol, aldehydes and cyclic 1,3-dicarbonyl compounds, Tetrahedron Lett. 49 (2008) 7117-7120. |
| [31] | N. Foroughifar, A. Mobinikhaledi, H. Moghanian, A catalytic and green procedure for synthesis of 12-aryl- or 12-alkyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11- one derivatives under solvent-free conditions, Int. J. Green Nanotechnol. Phys. Chem. 1 (2009) 57-63. |
| [32] | K. Tabatabaeian, A. Khorshidi, M. Mamaghani, A. Dadashi, M.K. Jalali, One-pot synthesis of tetrahydrobenzo[a]xanthen-11-one derivatives catalyzed by ruthenium chloride hydrate as a homogeneous catalyst, Can. J. Chem. 89 (2011) 623-627. |
| [33] | A. Zarei, A.R. Hajipour, L. Khazdooz, The one-pot synthesis of 14-aryl or alkyl-14Hdibenzo[a,j] xanthenes catalyzed by P2O5/Al2O3 under microwave irradiation, Dyes Pigm. 85 (2010) 133-138. |
| [34] | B.B. Mirjalili, A. Bamoniri, A. Akbari, BF3·SiO2: an efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]xanthenes, Tetrahedron Lett. 49 (2008) 6454-6456. |
| [35] | N.D. Kokare, J.N. Sangshetti, D.B. Shinde, Oxalic acid as a catalyst for efficient synthesis of bis-(indolyl)methanes, and 14-aryl-14H-dibenzo[a,j]xanthenes in water, Chin. Chem. Lett. 19 (2008) 1186-1189. |
| [36] | F. Shirini, N.G. Khaligh, G.H. Imanzadeh, P.G. Ghasem-Abadi, 1,3-Dibromo-5,5- dimethylhydantoin (DBH)/kaolin: an efficient reagent system for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions, Chin. Chem. Lett. 23 (2012) 1145-1148. |
| [37] | J. Safaei-Ghomi, M.A. Ghasemzadeh, Zinc oxide nanoparticles: a highly efficient and readily recyclable catalyst for the synthesis of xanthenes, Chin. Chem. Lett. 23 (2012) 1225-1229. |
| [38] | B. Das, B. Ravikanth, R. Ramu, K. Laxminarayana, B.V. Rao, Iodine catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo[a,j]xanthenes, J. Mol. Catal. A: Chem. 255 (2006) 74-77. |
| [39] | A.P. Mohamed, P.J. Vaderapura, Molecular iodine catalyzed synthesis of aryl-14Hdibenzo[a,j]xanthenes under solvent-free condition, Bioorg. Med. Chem. Lett. 17 (2007) 621-623. |
| [40] | M. Mohammadpour Amini, M. Seyyedhamzeh, A. Bazgir, Heteropolyacid: an efficient and eco-friendly catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]- xanthene, Appl. Catal. A: Gen. 323 (2007) 242-245. |
| [41] | M.M. Heravi, Kh. Bakhtiari, Z. Daro@gheha, F.F. Bamoharram, Facile heteropolyacid- promoted synthesis of 14-substituted-14-H-dibenzo[a,j] xanthene derivatives under solvent-free conditions, J. Mol. Catal. A: Chem. 273 (2007) 99-101. |
| [42] | H.R. Shaterian, M. Ghashang, A. Hassankhani, One-pot synthesis of aryl 14Hdibenzo[a,j]xanthene leuco-dye derivatives, Dyes Pigm. 76 (2008) 564-568. |
| [43] | M. Seyyedhamzeh, P. Mirzaei, A. Bazgir, Solvent-free synthesis of aryl-14Hdibenzo[a,j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst, Dyes Pigm. 76 (2008) 836-839. |
| [44] | S. Ko, C.F. Yao, Heterogeneous catalyst: amberlyst-15 catalyzes the synthesis of 14-substituted-14H-dibenzo[a,j]xanthenes under solvent-free conditions, Tetrahedron Lett. 47 (2006) 8827-8829. |
| [45] | B.F. Mirjalili, A. Bamoniri, A. Akbari, N. Taghavinia, Nano-TiO2: an eco-friendly and re-usable catalyst for the synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]- xanthenes, J. Iran. Chem. Soc. 8 (2011) S129-S134. |
| [46] | M. Dabiri, M. Baghbanzadeh, M.S. Nikcheh, E. Arzroomchilar, Eco-friendly and efficient one-pot synthesis of alkyl- or aryl-14H-dibenzo[a,j]xanthenes in water, Bioorg. Med. Chem. Lett. 18 (2008) 436-438. |
| [47] | H.J. Zang, Y. Zhang, B.W. Cheng, Efficient one-pot synthesis of 12-aryl-8,9,10,12- tetrahydrobenzo[a]xanthen-11-one derivatives using NaHSO4 as catalyst in ionic liquid, Adv. Mater. Res. 113 (2010) 1993-1996. |
| [48] | A. Sharifi, M.S. Abaee, A. Tavakkoli, M. Mirzaei, A. Zolfaghari, Facile montmorillonite k-10-supported synthesis of xanthene derivatives under microwave and thermal conditions, Synth. Commun. 38 (2008) 2958-2966. |
| [49] | A.R. Khosropour,M.M. Khodaei, H.Moghannian, A facile, simple and convenient method for the synthesis of 14-alkyl or aryl-14-H-dibenzo[a,j]xanthenes catalyzed by pTSA in solution and solvent-free conditions, Synlett (2005) 955- 958. |
| [50] | N.A. Bigdeli, M.M. Heravi, G.H. Mahdavinia, Wet cyanuric chloride catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo[a,j]xanthenes, Catal Commun. 8 (2007) 1595-1598. |
| [51] | S. Allameh, A. Davoodnia, A. Khojastehnezhad, An efficient and eco-friendly synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using H4[SiW12O40] as a heterogeneous and reusable catalyst under solvent-free conditions, Chin. Chem. Lett. 23 (2012) 17-20. |
| [52] | W. Su, D. Yang, C. Jin, B. Zhang, Yb(OTf)3 catalyzed condensation reaction of β-naphthol and aldehyde in ionic liquids: a green synthesis of aryl-14H-dibenzo[a,j]xanthenes, Tetrahedron Lett. 49 (2008) 3391-3394. |
| [53] | F. Shirini, N.G. Khaligh, Succinimide-N-sulfonic acid: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions, Dyes Pigm. 95 (2012) 789-794. |
| [54] | F. Shirini, N.G. Khaligh, S. Akbari-Dadamahaleh, Preparation, characterization and use of 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient, halogenfree and reusable ionic liquid catalyst for the trimethylsilyl protection of hydroxyl groups and deprotection of the obtained trimethylsilanes, J. Mol. Catal. A: Chem. 365 (2012) 15-23. |
| [55] | F. Shirini, N.G. Khaligh, 1,3-Disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable ionic liquid catalyst for the N-Boc protection of amines, J. Mol. Liq. 177 (2013) 386-393. |
| [56] | L. Khazdooz, A. Zarei, A.R. Hajipour, N. Sheikhan, A study for the synthesis of dibenzo [a,j] xanthenes and 1-amidoalkyl 2-naphthols catalyzed by [Hmim][HSO4] as a green, efficient and reusable catalyst under solvent-free conditions, Iran. J. Catal. 1 (2011) 1-10. |
| [57] | G.H. Mahdavinia, S. Rostamizadeh, A.M. Amani, Z. Emdadi, Ultrasound-promoted greener synthesis of aryl-14-H-dibenzo[a,j]xanthenes catalyzed by NH4H2PO4/ SiO2 in water, Ultrason. Sonochem. 16 (2009) 7-10. |
| [58] | B. Maleki, M. Gholizadeh, Z. Sepehr, 1,3,5-Trichloro-2,4,6-triazinetrion: a versatile heterocycle for the one-pot synthesis of 14-aryl- or alkyl-14H-dibenzo[a,j]- xanthene, 1,8-dioxooctahydroxanthene and 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives under solvent-free conditions, Bull. Korean Chem. Soc. 32 (2011) 1697-1702. |
| [59] | H. Hashemi, A.R. Sardarian, 4-Dodecylbenzenesulfonic acid (DBSA) as an efficient and recyclable catalyst for synthesis of 14-aryl- and 14-alkyl-14-Hdibenzo[a,j]xanthenes under solvent-free conditions, Iran. J. Sci. Technol. A1 (2013) 75-82. |
| [60] | A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, et al., Organocatalyst trityl chloride efficiently promoted the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones by in situ formation of carbocationic system in neutral media, Catal. Commun. 20 (2012) 54-57. |
| [61] | G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, An efficient one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under solvent free condition, Tetrahedron 65 (2009) 7129-7134. |
| [62] | M.M. Heravi, H. Alinejhad, K. Bakhtiari, H.A. Oskooie, Sulfamic acid catalyzed solvent-free synthesis of 10-aryl-7,7-dimethyl-6,7,8,10-tetrahydro-9H-[1,3]- dioxolo [4,5-b]xanthen-9-ones and 12-aryl-9,9-dimethyl-8,9,10,12-tetrahydro- 11H-benzo[a]xanthen-11-ones, Mol. Divers. 14 (2010) 621-626. |
| [63] | F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, A.R. Aliakbar, Rice husk: a mild, efficient, green and recyclable catalyst for the synthesis of 12-aryl- 8,9,10,12-tetrahydro[a]xanthene-11-ones and quinoxaline derivatives, C.R. Chim. 16 (2013) 207-216. |
| [64] | K. Venkatesan, S.S. Pujari, R.J. Lahoti, K.V. Srinivasan, An efficient synthesis of 1,8- dioxo-octahydro-xanthene derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation, Ultrason. Sonochem. 15 (2008) 548-553. |
| [65] | S. Kokkirala, N.M. Sabbavarapu, V.D.N. Yadavalli, β-Cyclodextrin mediated synthesis of 1,8-dioxooctahydroxanthenes in water, Eur. J. Chem. 2 (2011) 272-275. |
| [66] | A. Zare, A.R. Moosavi-Zare, M. Merajoddin, et al., Ionic liquid triethylaminebonded sulfonic acid {[Et3N-SO3H]Cl} as a novel, highly efficient and homogeneous catalyst for the synthesis of β-acetamido ketones, 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a,j]xanthenes, J. Mol. Liq. 167 (2012) 69-77. |
| [67] | S. Rostamizadeh, A.M. Amani, G.H. Mahdavinia, G. Amiri, H. Sepehrian, Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media, Ultrason. Sonochem. 17 (2010) 306-309. |
| [68] | M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, et al., Preparation of various xanthene derivatives over sulfonic acid functionalized imidazolium salts (SAFIS) as novel, highly efficient and reusable catalysts, C.R. Chim. 15 (2012) 719-736. |
| [69] | K. Gong, D. Fang, H.L. Wang, X.L. Zhou, Z.L. Liu, The one-pot synthesis of 14-alkylor aryl-14H-dibenzo[a,j]xanthenes catalyzed by task-specific ionic liquid, Dyes Pigm. 80 (2009) 30-33. |
| [70] | D. Kundu, A. Majee, A. Hajra, Task-specific ionic liquid catalyzed efficient microwave- assisted synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen- 11-ones under solvent-free conditions, Green Chem. Lett. Rev. 4 (2011) 205-209. |
| [71] | B. Janardhan, S. Vijaya Laxmi, B. Rajitha, An efficient synthesis of 12-aryl- 8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones using (4-sulfobutyl)tris(4-sulfophenyl) phosphonium hydrogen sulphate as catalyst under neat conditions, J. Chem. Pharm. Res. 4 (2012) 519-525. |
| [72] | J.M. Khurana, D. Magoo, pTSA-catalyzed one-pot synthesis of 12-aryl-8,9,10,12- tetrahydrobenzo[a]xanthen-11-ones in ionic liquid and neat conditions, Tetrahedron Lett. 50 (2009) 4777-4780. |
| [73] | A. Zare, R. Khanivar, M. Hatami, et al., Efficient synthesis of 12-aryl-8,9,10,12- tetrahydrobenzo[a]-xanthen-11-ones using ionic liquid pyrazinium di(hydrogen sulfate) {Py(HSO4)2} as a novel, green and homogeneous catalyst, J. Mex. Chem. Soc. 56 (2012) 389-394. |
| [74] | A. Zare, R. Khanivar, M. Merajoddin, et al., Triethylamine-bonded sulfonic acid {[Et3N-SO3H]Cl} as an efficient and homogeneous catalyst for the synthesis of 12- aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones, Iran. J. Catal. 2 (2012) 107- 114. |
| [75] | K. Niknam, M. Damya, 1-Butyl-3-methylimidazolium hydrogen sulfate[bmim]HSO4: an efficient reusable acidic ionic liquid for the synthesis of 1,8- dioxo-octahydroxanthenes, J. Chin. Chem. Soc. 56 (2009) 659-665. |
| [76] | M. Dabiri, M. Baghbanzadeh, E. Arzroomchilar, 1-Methylimidazolium triflouroacetate ([Hmim]TFA): an efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines, Catal. Commun. 9 (2008) 939. |
| [77] | M. Kalantari, Synthesis of 1,8-dioxo-octahydroxanthenes and bis(indolyl)- methanes catalyzed by [Et3NH][H2PO4] as a cheap and mild acidic ionic liquid, Arabian J. Chem. 5 (2012) 319-323. |