Spiro heterocycles often exist in a number of natural or synthetic molecules [1, 2, 3]. A spiro compound in which the spiro carbon is part of cyclic ring has many unique properties,such as spiroconjugation,anomeric effect and axial chirality [4, 5, 6, 7]. Therefore,spiro heterocyclic scaffolds have broad applications in many areas [8, 9, 10, 11, 12]and are widely used as building blocks for generating biological and pharmaceutical relevance [13, 14, 15].
Neonicotinoid is the largest insecticide now [16, 17, 18],but its superiority is being challenged due to resistance [19] and severe bee toxicity [20, 21]. Thus,there is an urgent need for the development of novel,effective,neonicotinoid replacements. As part of a project aiming at exploring functionality of the spiro heterocycle core in neonicotinoids,we herein report our investigation in spiro heterocycle containing neonicotinoids.
Melting points (mp) were recorded on Büchi B540 apparatus (Büchi Labortechnik AG,Flawil,Switzerland) and are uncorrected. The 1HNMR and 13CNMR spectra were recorded on Bruker AM-400 (400 MHz) spectrometer with DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in d (parts per million) values. High-resolution mass spectra were recorded under electron impact (70 eV) conditions using a MicroMass GCT CA 055 instrument. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254),and spots were visualized with ultraviolet (UV) light.
The general synthetic methods for compounds 8a-d,9a-g and 10a and 10b are depicted in Schemes 1-3. Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Yields were not optimized. All reactions were carried out under a protective atmosphere of drying nitrogen or utilizing a calcium chloride tube.

|
Download:
|
| Scheme 1.Synthetic routes for neonicotinoids containing spiro heterocycles 8a-d. | |

|
Download:
|
| Scheme 2.Synthetic routes for neonicotinoids containing spiro heterocycles 9a-g. | |
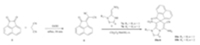
|
Download:
|
| Scheme 3.Synthetic routes for neonicotinoids containing spiro heterocycles 10a-b. | |
General synthetic procedure for 8a-d: Knoevenagel adduct 2 was synthesized according to the reported procedure [8]. A mixture of phthalic anhydride (1.480 g,10mmol) and malononitrile (0.66 g, 11mmol) in THF (25mL) were stirred at room temperature. Then, diisopropylamine (DIPA) (2.02 g,20mmol) was added dropwise. The resulting mixture was stirred at room temperature for 8 h,and then was filtered and the precipitate was washed with THF (10 mL) to afford the white solid. Then,to a solution of the obtained white solid in 1,2-dichloroethane (20 mL) was added POCl3 (10 mmol) dropwise at room temperature. After completion,the mixture was refluxed and the progress of the reaction was monitored by TLC. At last,the reactionmixture was neutralized by saturated dicarbonate solution to pH 7,extracted by dichloromethane (30 mL × 3), concentrated and the residuewas purified by flash chromatography eluting with dichloromethane to afford compound 2 (yield 40%). Then,a solution of compound 2 (0.190 g,1 mmol) and compounds 7a-d (1 mmol) in DMF (4 mL) was heated at 50℃ or 120℃ with the progress of the reaction monitored by TLC. After completion,the mixture was concentrated and the residue was purified by recrystallization with ethanol to afford the pure products 8a-d.
General synthetic procedure for 9a-g: Method 1 (for 9a,9b and 9g): A mixture of isatin (1.470 g,10 mmol),malononitrile (0.60 g, 10 mmol) and piperidine (10 mol%) in absolute ethanol (10 mL) was refluxed for 1 h. The cooled mixture was filtered and the precipitate was washed with ethanol (4 mL) to afford compound 4 (yield 80%). Then,a solution of compound 4 (0.189 g,1 mmol) and compound 7a-c (1 mmol) in ethanol (4 mL) was refluxed for 8 h. The mixture was filtered and the precipitate washed with cold ethanol (4 mL) affording the pure products 9a,9b and 9g. Method 2 (one-pot procedure for 9c-9f): A solution of 3b or 3c (1 mmol), malononitrile (0.060 g,1 mmol) and piperidine (10 mol%) was refluxed in ethanol (4 mL) for 1 h. Then,7a or 7b was added simultaneously. The resulted mixture was stirred for 8 h under refluxing condition with the progress of the reaction monitored by TLC. After completion,the cooled mixture was filtered and the precipitate was washed with cold ethanol (4 mL) to afford the desired products 9c-9f.
General synthetic procedure for 10a and 10b: A mixture of acenaphthylene-1,2-dione (1.820 g,10 mmol) and malononitrile 0.60 g,10 mmol) in absolute ethanol (20 mL) was refluxed for 30 min. The cooled mixture was filtered and the precipitate was washed with cold ethanol (10 mL) to afford compound 6 (yield 85%). Then,a solution of compound 6 (0.230 g,1 mmol) and 7a and 7b (1 mmol) in 5 mL of mixed solvent of dichloromethane and methanol (4:1,v/v) was stirred for 2 h at room temperature. At last, the mixture was filtered and the precipitate was washed with methanol (4 mL) to afford the pure products 10a and 10b.
Analytical data of the target compounds are listed in Supporting information.
All bioassays were performed on representative test organisms grown in the laboratory. The bioassay was repeated at (25 ± 1)℃ according to statistical requirements. All compounds were dissolved in N,N-dimethylformamide (AP,Shanghai Chemical Reagent Co.,Ltd., Shanghai,China) and diluted with distilled water containing Triton X- 100 (0.1 mg L-1) to obtain a series of concentrations of 500.0,250.0, 125.0 mg L-1 and others for bioassays. The results of bioassay are depicted in Table 1.
| Table 1 Oligonucleotides designed in the present study. |
Insecticidal test for cowpea aphids (Aphis craccivora): The activities of insecticidal compounds against cowpea aphids were tested by leaf-dip method. The leaves of the horsebean plant with 40-60 apterous adults were dipped in diluted solutions of the chemicals containing Triton X-100 (0.1 mg L-1) for 5 s and the excess solution was sucked out with filter paper,and the burgeons were placed in the conditioned room ((25 ±1)℃,50% RH). Water containing Triton X-100 (0.1 mg L-1) was used as a control. The mortality rates were evaluated 24 h after treatment. Each treatment had three repetitions and the data were adjusted and subjected to probit analysis as before.
Insecticidal test for Armyworm (Mythimna separata): The insecticidal activity against armyworm was tested by foliar application. Individual corn (Zea mays) leaves were placed on moistened pieces of filter paper in Petri dishes. The leaves were then sprayed with the sample solutions and exposed to dry. The dishes were infested with 10 s-instar larvae and placed in the conditioned room. The mortality rates were evaluated 48 h after treatment. Each treatment had three repetitions and the data were adjusted and subjected to probit analysis as before.
Insecticidal test for brown planthopper (Nilaparvata lugens): The activities of insecticidal compounds against Nilaparvata lugens were tested using the dipping method. Rice plants at tillering to booting stage were pulled out and the rice stems (about 10 cm lengths) with roots were cut and air dried to remove excess water. Three rice stems were dipped in appropriate solutions of tested compound for 30 s. After rice stems had been air dried,the rice roots was wrapped with moistened cotton and then placed into a tumbler. Thirty N. lugens were introduced into the tumbler with the treated insects were maintained at a temperature of (27 ±1)℃. Only distilled water only was used as control for each tested chemical. Each process was repeated for 3 times and the mortality rates were evaluated 48 h after treatment. The data were adjusted and subjected to probit analysis as before.
The introduction of a spiro heterocycle into neonicotinoid molecule was accomplished by reactions of nitroenamine 7,a neonicotinoid precusor,and Knoevenagel adducts (KAs) 2,4 and 6. Nitroenamines have highly polarized push-pull ethylene systems, making them reactive intermediates with a vast variety of electrophiles [22, 23, 24]. In a previous study,we found that nitroenamine could react with KAs benzylidenemalononitriles affording tetrahydroimidazo[1,2-a]pyridine derivatives as high active neonicotinoids [25]. Enlightened by this,the spiro heterocycle might be embodied in parental neonicotinoid by the corresponding spirocyclization using cyclic KAs. Therefore,cyclic KAs 2,4 and 6 were prepared here by condensation of malononitrile and cyclic ketones according to reported procedures [8, 26].
Reactions of 7 with KAs 2,4 and 6 afforded spirobenzofuranones 8a-d,spirooxindoles 9a-g and spiroaceraphthylenones 10a and 10b,respectively,as spiro heterocycle containing neonicotinoids. In further studies,we found that compounds 9a-g could be easily obtained in high yields by a one-pot method from nitroeamine 7, ketone 3 and malononitrile. The reaction solvents of 7a with 6 were screened. The reaction proceeded very well in the mixed solvent of dichloromethane and methanol (4:1),while no reactions were detected in the single solvents,such as dichloromethane,methanol, ethanol,pyridine and acetonitrile. Spirocyclization of 7c with 6 was unsuccessful probably due to steric effects. Attempts to synthesize corresponding products using other cyclic KAs 2-(2,3- dihydro-1H-inden-1-ylidene)malononitrile and 2-cyclohexylidenemalononitrile failed in varied solvents,such as acentonitrile, dichloromethane,methanol and DMF.
The insecticidal activity of the target compounds against cowpea aphids (A. craccivora),armyworm (M. sepatara) and brown planthopper (N. lugens)was evaluated. Spirobenzofuranone analogs 8a,8b and 8d have some activity against armywormwith 100%,20% and 80% mortality,respectively,while 8c showed moderate activity to hemiptera insects such as cowpea aphids and brownplanthopper. The other two types of spiro heterocyclic neonicotinoids 9a-g and 10a and 10b were inactive to these three tested insects. The poor activity of these compounds might be caused by their lowsolubility in water and the large size of the spirocycles whichmade it difficult to bind within the receptor pocket.
The first introduction of a spiro heterocycle into the neonicotinoid molecule was accomplished. Using cyclic KAs,neonicotinoids bearing spirobenzofuranone,spirooxindole and spiroacenaphythylenone were successfully constructed and their insecticidal activity was screened. Some compounds had moderate insecticidal activity,and ongoing research is focused on improving the efficacy through further analog generation. These novels compounds are expected to provide a basis for designing new spiro heterocycle containing neonicotinoids.
This work was financial supported by National Basic Research Program of China (973 Program,No. 2010CB126100),National High Technology Research Development Program of China (863 Program,No. 2011AA10A207),Key Projects in the National Science & Technology Pillar Program (No. 2011BAE06B05),National Natural Science Foundation of China (No. 21372079),Shanghai Education Committee (No. 12ZZ057) and the Fundamental Research Funds for the Central Universities. This work was also partly supported by Australia DC Foundation.
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.12.004.
| [1] | R. Rios, Enantioselective methodologies for the synthesis of spiro compounds, Chem. Soc. Rev. 41 (2012) 1060-1074. |
| [2] | T. Jin, M. Himuro, Y. Yamamoto, Triflic acid catalyzed synthesis of spirocycles via acetylene cations, Angew. Chem. Int. Ed. 48 (2009) 5893-5896. |
| [3] | G.S. Singh, Z.Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks, Chem. Rev. 112 (2012) 6104-6155. |
| [4] | H. Diirr, R. Gleiter, Spiroconjugation, Angew. Chem. Int. Ed. 17 (1978) 559-569. |
| [5] | B. Gleiter, H. Hoffmann, H. Irngartinger, M. Nixdorf, Donor-acceptor spiro-compounds- syntheses, structures and electronic properties, Chem. Ber. 127 (1994) 2215-2224. |
| [6] | J. Sun, Y.J. Xie, C.G. Yan, Construction of dispirocyclopentanebisoxindoles via selfdomino michael-aldol reactions of 3-phenacylideneoxindoles, J. Org. Chem. 78 (2013) 8354-8365. |
| [7] | K. Murai, H. Komatsu, R. Nagao, H. Fujioka, Oxidative rearrangement of spiro cyclobutane cyclic aminals: efficient construction of bicyclic amidines, Org. Lett. 14 (2012) 772-775. |
| [8] | W.Y. Xu, Y.M. Jia, J.K. Yang, Z.T. Huang, C.Y. Yu, Reactions of heterocyclic detene aminals with 2-[3-oxoisobenzofuran-1(3H)-ylidenne]malononitrile: synthesis of novel polyfunctionalized 1,4-dihydropyridine-fused 1,3-diazaheterocycles, Synlett 11 (2010) 1682-1684. |
| [9] | F. Shi, G.J. Xing, R.Y. Zhu, W. Tan, S.J. Tu, A catalytic asymmetric isatin-involved povarov reaction: diastereo- and enantioselective construction of spiro[indolin- 3,2'-quinoline] scaffold, Org. Lett. 15 (2013) 1128-1131. |
| [10] | S. Pal, M.N. Khan, S. Karamthulla, S.J. Abbas, L.H. Choudhury, One pot fourcomponent reaction for the efficient synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate derivatives, Tetrahedron Lett. 54 (2013) 5434-5440. |
| [11] | F.Y. Miyake, K. Yakushijin, D.A. Horne, Preparation and synthetic applications of 2-halotryptamines: synthesis of elacomine and isoelacomine, Org. Lett. 6 (2004) 711-713. |
| [12] | B. Tan, N.R. Candeias, C.F. Barbas Ⅲ Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst, Nat. Chem. 3 (2011) 473-477. |
| [13] | S. Rapposelli, M.C. Breschi, V. Calderone, et al.,A. Balsamo, Synthesis and biological evaluation of 5-membered spiro heterocycle-benzopyran derivatives against myocardial ischemia, Eur. J. Med. Chem. 46 (2011) 966-973. |
| [14] | C.W. Lee, R. Lira, J. Dutra, K. Ogilvie, et al., Stereoselective synthesis of spiropiperidines as BACE-1 aspartyl protease inhibitors via late stage N-arylation of a 1,8-diazaspiro[4.5]dec-3-en-2-one pharmacophore, J. Org. Chem. 78 (2013) 2661-2669. |
| [15] | M. Rottmann, C. McNamara, B.K.S. Yeung, et al., Spiroindolones, a potent compound class for the treatment of malaria, Science 329 (2010) 1175-1180. |
| [16] | A. Elbert, M. Schindler, R. Nauen, P. Jeschke, Overview of the status and global strategy for neonicotinoids, J. Agric. Food Chem. 59 (2011) 2897-2908. |
| [17] | S. Kagabu, Discovery of imidacloprid and further developments from strategic molecular designs, J. Agric. Food Chem. 59 (2011) 2887-2896. |
| [18] | M. Tomizawa, J.E. Casida, Molecular recognition of neonicotinoid insecticides: the determinants of life or death, Acc. Chem. Res. 42 (2009) 260-269. |
| [19] | R. Nauen, I. Denholm, Resistance of insect pests to neonicotinoid insecticides: current status and future prospects, Arch. Insect. Biochem. Physiol. 58 (2005) 200-215. |
| [20] | M. Henry, M. Bé guin, F. Requier, et al., A common pesticide decreases foraging success and survival in honey bees, Science 336 (2012) 348-350. |
| [21] | S.A. Cameron, J.D. Lozier, J.P. Strange, et al., Patterns of widespread decline in North American bumble bees, Proc. Natl. Acad. Sci. U.S.A. 108 (2011) 662-667. |
| [22] | X.S. Shao, P.W. Lee, Z.W. Liu, et al., cis-Configuration: a new tactic/rationale for neonicotinoid molecular design, J. Agric. Food Chem. 59 (2011) 2943-2949. |
| [23] | X.S. Shao, Z. Li, X.H. Qian, X.Y. Xu, Design, synthesis and insecticidal activities of novel analogues of neonicotinoids: replacement of nitromethylene with nitroconjugated system, J. Agric. Food Chem. 57 (2009) 951-957. |
| [24] | X.S.Shao,H.Fu, X.Y.Xu, etal.,Divalentandoxabridgedneonicotinoidsconstructedby dialdehydes and nitromethylene analogues of imidacloprid: design, synthesis, crystal structure, and insecticidal activities, J. Agric. Food Chem. 58 (2010) 2696-2702. |
| [25] | W.W. Zhang, X.B. Yang, W.D. Chen, et al., Design, multicomponet synthesis, and bioactivities of novel neonicotinoid analogues with 1,4-dihydropyridine scaffold, J. Agric. Food Chem. 58 (2010) 2741-2745. |
| [26] | A. Alizadeh, T. Firuzyar, A. Mikaeili, Efficient one-pot synthesis of spirooxindole derivatives containing 1,4-dihydropyridine-fused-1,3-diazaheterocycle fragments via four-component reaction, Synthesis 22 (2010) 3913-3917. |