b Research & Development (API) Centre, Panacea Biotech Ltd., B-1 Extn./A-27, Mohan Co-operative Industrial Estate, New Delhi 110 044, India
The α-bromination of ketones has attracted much attention because the resulting α-bromination of ketones are the key intermediates in organic synthesis [1, 2]. Accordingly, a number of protocols have been developed which include cupric bromide [3], dioxane dibromide [4], tetrα-butyl ammonium tribromide [5], H2O2-HBr [6], bromodimethyl sulfoniumbromide [7], ethylene bis(N-methyl imidazolium) ditribromide [8], trihaloisocyanuric acids [9], pyridinium bromochromate [10] and NH4Br-oxone [11]. Moreover, owing to user friendliness and availability, N-bromosuccinimide has been extensively used as brominating agent: transformations can be modulated under various reaction conditions, for example: (i) using different types of catalysts such as Mg(ClO4)2 [12], NH4OAc [13], Amberlyst-15 [14], silicα-supported NaHSO4 [15], silica supported NaHCO3 [16], sulfonic acid functionalized silica [17] and FeCl3 [18]; (ii) ionic liquids [19], (iii) photochemical bromination [20]; (iv) sonochemical bromination [21] and (v) solvent free reaction conditions (SFRC) [22].
An extensive literature search revealed that bromination with NBS depends on the catalyst used and this encouraged researchers to develop a number of catalysts to make the bromination process easier and more efficient. Silica gel is known as a good catalyst in many synthetic applications [23, 24] and also for ring bromination [25, 26, 27]; However, no reports were found on the investigation of silica gel as catalyst for the α-bromination of ketones using NBS. As a part of continuing agenda to explore the use of inorganic materials in the synthesis of organic compounds, we found that silica gel is an efficient catalyst for the α-bromination of ketones when N-bromosuccinimide in methanol is used under reflux conditions (Scheme 1).

|
Download:
|
| Scheme 1.The α-bromination of ketones using NBS in presence of silica gel in methanol. | |
Ketones were purchased from Acros, Merck and S.D. Fine Chemicals Ltd., Mumbai, India. N-bromosuccinimide and silica gel (60-120 mesh) were procured from Merck, India. Methanol (99.0%) and ethyl acetate were purchased from Finar Chemicals Ltd., India. Double distilled water was used for work-up. Melting points were determined in open capillaries on a Mel-Temp apparatus and are uncorrected. The 1H NMR spectra were recorded on a Varian 300 MHz. Chemical shifts were expressed in parts per million (ppm). Splitting patterns describe apparent multiplicities and are designated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) or br (broad). Mass spectra (MS) were recorded on Agilent, model-6410, triple quad LCMS. Thin-layer chromatography was performed using 0.25 mm Merck silica gel plates (60F- 254) and visualized with UV light. Column chromatography was performed on silica gel of 60-120 mesh, Merck).
Column chromatographic grade silica gel (60-120 mesh) supplied by Merck, India was employed in the present investigation. The pH value of 10% aqueous suspension of silica gel is ~6.5- 7.5. Silica gel catalyst was used as it is, without any activation or any further treatment or chemical modifications.
The α-bromination reaction was carried out using acetophenone (1200 mg, 10 mmol), N-bromosuccinimide (2136 mg, 12 mmol), 10% (w/w) silica gel (120 mg) in 10 mL of methanol at reflux conditions until the disappearance of the substrate. (Note: 2136 mg of N-bromosuccinimide was added portion wise i.e. 356 mg for each time in six portions). The progress of the reaction was monitored by TLC. The reaction mass was filtered after the completion of the reaction as per TLC and the catalyst was collected for reuse. The filtrate was concentrated under vacuum. Double distilled water was added to the reaction mixture and quenched with aqueous sodium thiosulfate and the product extracted with dichloromethane (Caution: Severe burning sensation of eyes was observed during the work-up process). The layers were separated and the organic layer was collected and washed thrice with distilled water (3 × 50 mL). The collected organic layer was dried over anhydrous Na2SO4, filtered and concentrated. The obtained crude product was purified by column chromatography over silica gel (60-120 mesh) using n-hexane-EtOAc (99:1 ratio).
With the aim of studying the recycling of the catalyst, the isolated catalyst was washed with ethyl acetate (5 mL) after its filtration from the reaction medium, collected and dried in vacuum at 70 ℃ to a constant weight. Subsequently it was reused for the abromination of acetophenone and achieved 95%, 86% and 83% yields of product (2a) for first, second and third reuse of catalyst respectively. All products gave spectroscopic data in agreement with the literature [15, 21, 27, 28, 29, 30].
The method is also very practical for scale up in process development. We attempted large scale (100 gram scale) synthesis of 2-bromo-1-phenylethanone 2a and obtained fruitful results with isolated yields ranging from 93% to 96%.
The α-bromination reaction was carried out using acetophenone (100 g, 0.832 mol), N-bromosuccinimide (213.6 g, 1.2 mol), 10% (w/w) silica gel (10 g) in 1 L of methanol under reflux conditions until the disappearance of the substrate (Note: 213.6 g of N-bromosuccinimide was added portion wise i.e. 35.6 g for each time in six portions). Excess N-bromosuccinimide (+0.1 mol) was added to complete the reaction as per TLC. The reaction mass was filtered, after the completion of the reaction as per TLC and the catalyst was collected for reuse. The solvent was removed and double distilled water was added to the reaction mixture and was quenched with aqueous sodium thiosulfate. The product was extracted with dichloromethane. (Caution: Severe burning sensation of eyes was observed during the work-up process). The layers were separated and the organic layer was collected and it was washed with distilled water for three times (3 × 1 L). The collected organic layer was dried over anhydrous Na2SO4 and filtered, concentrated and purified by recrystallization using n-hexane (300 mL). The resulting yield of pure α-brominated ketone, i.e. 2- bromo-1-phenylethanone (2a) was 94%. The same experiment was repeated twice and the yields were 93% and 96%, respectively. The reproducibility of the protocol was thus confirmed with the consistent isolated yields of desired product 2-bromo-1-phenylethanone (2a).
To study the effect of solvents and temperature on the abromination of ketones, we carried out a reaction with acetophenone using N-bromosuccinimide in methanol in presence of silica gel at room temperature. However a low yield (56%, Table 1 entry 1) of the product 2-bromo-1-phenylethanone (2a) was obtained after 4 h. When the same reaction was carried out under reflux conditions (Table 1, entry 1), the yield was 95% within 18 min. Other solvents such as Et2O, THF, CH3CN, CH2Cl2 and CHCl3 were also studied, but lower yields of the desired products were obtained (10-35%) as shown in Table 1. With Et2O, THF, 1,4- dioxane and acetonitrile, lower yields of the product 2a was formed, along with very low amounts of the α, α'-dibrominated product (3a) and interestingly, the nuclear brominated product 4a was formed as the major product (Table 1, entries 2-4). In chlorinated solvents, the formation of the desired product 2a was very low. This may be attributed to the lack of solubility of NBS in these solvents (Table 1, entries 6-7). The reaction did not proceed in the absence of catalyst (Table 1, entry 8). Solvent screening experiments revealed that methanol was the best solvent for the silica gel catalyzed α-bromination. For further studies, methanol was used as a solvent to optimize other reaction parameters.
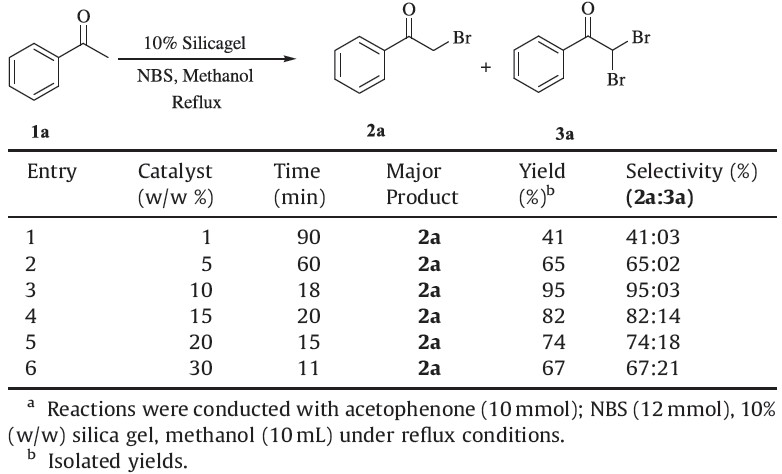
We studied the time course of the α-bromination reaction and subsequent effect on yield of the product (2a) using acetophenone as a model substrate in the presence of different weight percentage (w/w) of silica gel in methanol under reflux conditions with the acquired results displayed in Table 2. When 1% of catalyst was used, 41% yield of the product (2a) was obtained in 90 min. Upon use of 5% of catalyst, a 65% yield was observed in 60 min and with 10% of catalyst, the obtained yield reached the maximum of 95% in 18 min On the other hand with 15, 20 and 30% of catalyst, 82, 74 and 67% yields were obtained, respectively and the reaction was completed within 10-15 min. Low catalyst load resulted in the existence of high percentage of unreacted substrate (Table 2, entries 1 and 2) even with long reaction times. In contrast, an increase of catalyst load caused a decrease in the isolated yield of the desired product 2a and a subsequent increase of α,α’-dibrominated product (3a) formation (Table 2, entries 4-6), but the reaction was rapidly completed.
To further examine the selectivity of this silica gel catalyzed α-bromination reaction, different substituted acetophenones, acenaphthones, acyclic and cyclic ketones were employed. In the α-bromination reaction of acetophenone, a high yield (95%) of product (2a) was observed (Table 3, entry 1). The α-bromination of substituted acetophenones gave moderate to high yields, regardless of the electronic influence of substituents (Table 3, entries 2- 13), with the exception of m-nitroacetophenone, p-nitroacetophenone and p-cyanoacetophenone (Table 3, entries 11-13) wherein low yields of products 2k, 2l and 2m were obtained respectively. This may be due to the presence of strong electron withdrawing groups on the aromatic ring. The products, 2k, 2l and 2m were stable during reaction conditions as per TLC, but showed only moderate stability under the work-up process. However, after isolation, compounds (2k-m) were preserved under cooling (0- 5 ℃) to prevent decomposition. Compounds (2k-m) were soluble both in methanol and dichloromethane. Acenaphthones without substitution gave the corresponding products 2n and 2o in 82 and 87% yield (Table 3, entries 14 and 15), respectively. The α-bromination of acyclic and cyclic ketones provided good yields of products within a short period of time (Table 3, entries 16-22).
| Table 1 Effect of solvent and temperature on the formation of a-brominated product (2a).a |
| Table 2 Role of catalyst load on the formation of a-brominated product (2a).a |
| Table 3 The α-bromination of various ketones using NBS in presence of silica gel.a |
In summary, a new method was developed using silica gel as a catalyst for the α-bromination of ketones (acyclic, cyclic and aralkyl ketones) in methanol utilizing NBS under reflux conditions. Thus, our new bromination reaction proceeded smoothly and afforded moderate to excellent isolated yields under optimized reaction conditions. The catalyst was efficiently recovered and reused for 3 times without loss of activity. Major advantages of the present protocol include shorter reaction times, user friendly process, simple work-up and exclusion of pre- and post-chemical treatment of catalyst, improved quality and appearance of the isolated products. In addition, the present method is highly useful for large scale (≥100 g scale) applications both in chemical and pharmaceutical industries.
We express our thanks to Council of Scientific and Industrial Research (CSIR), New Delhi, Government of India for the financial support under major research project (No. 01 (2391)/10/EMR-II).
| [1] | R.C. Larock, Comprehensive Organic Transformations, 2nd ed., Wiley-VCH, New York, (1999), p. 715. |
| [2] | For review see: A.W. Erian, S.M. Sherif, H.M. Gaber, The chemistry of α-haloketones and their utility in heterocyclic synthesis, Molecules 8 (2003) 93-865. |
| [3] | L.C. King, G.K. Ostrum, Selective bromination with copper (Ⅱ) bromide, J. Org. Chem. 29 (1964) 3459-3461. |
| [4] | S.J. Pasaribu, L.K. Williams, Selective bromination of substituted acetophenones with dioxan dibromide, Aust. J. Chem. 26 (1973) 1327-1331. |
| [5] | S. Kajigaeshi, T. Kakinami, T. Okamoto, S. Fujisaki, Synthesis of bromoacetyl derivatives by use of tetrabutylammonium tribromide, Bull. Chem. Soc. Jpn. 60 (1987) 1159-1160. |
| [6] | J. Ju, Y.J. Li, J.R. Gao, et al., High selectively oxidative bromination of toluene derivatives by the H2O2-HBr system, Chin. Chem. Lett. 22 (2011) 382-384. |
| [7] | A.T. Khan, M.A. Ali, P. Goswami, L.H. Choudhury, A mild and regioselective method for α-bromination of β-Keto esters and 1, 3-diketones using bromodimethylsulfonium bromide (BDMS), J. Org. Chem. 71 (2006) 8961-8963. |
| [8] | R. Hosseinzadeh, M. Tajbakhsh, M. Mohadjerani, Z. Lasemi, Ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB): an efficient reagent for the monobromination of 1,3-diketones and β-ketoesters, Monatsh. Chem. 140 (2009) 57-60. |
| [9] | G.F. Mendonça, H.C. Sindra, L.S. de Almeida, P.M. Esteves, M.C.S. de Mattos, Trihaloisocyanuric acids as convenient reagents for regioselective halogenation of β-dicarbonyl compounds, Tetrahedron Lett. 50 (2009) 473-475. |
| [10] | Y. Sarrafi, M. Sadatshahabi, K. Alimohammadi, A mild, simple and efficient method for selectiveα-monobromination of 1,3-diketones and β-keto-esters using pyridinium bromochromate, Chin. Chem. Lett. 20 (2009) 393-396. |
| [11] | M. Arun Kumar, C.N. Rohitha, M. Mahender Reddy, P. Swamy, N. Narender, Oxidative bromination of ketones using ammonium bromide and oxone, Tetrahedron Lett. 53 (2012) 191-195. |
| [12] | D. Yang, Y. Yan, B. Lui, Mildα-halogenation reactions of 1,3-dicarbonyl compounds catalyzed by Lewis acids, J. Org. Chem. 67 (2002) 7429-7431. |
| [13] | K. Tanemura, T. Suzuki, Y. Nishida, K. Satsumabayashi, T. Horaguchi, A mild and efficient procedure for α-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate, Chem. Commun. (2004) 470-471. |
| [14] | H.M. Meshram, P.N. Reddy, K. Sadashiv, J.S. Yadav, Amberlyst-15®-promoted efficient 2-halogenation of 1,3-keto-esters and cyclic ketones using N-halosuccinimides, Tetrahedron Lett. 46 (2005) 623-626. |
| [15] | B. Das, K. Venkateswarlu, G. Mahender, I. Mahender, A simple and efficient method for α-bromination of carbonyl compounds using N-bromosuccinimide in the presence of silica-supported sodium hydrogen sulfate as a heterogeneous catalyst, Tetrahedron Lett. 46 (2005) 3041-3044. |
| [16] | A. Rahman, S.B. Jonnalagadda, Simple and efficient system for the α-bromination of a β-ketoester by using N-bromosuccinimide in the presence of silica supported NaHCO3 as the heterogeneous catalyst: an environmentally benevolent approach, Synth. Commun. 42 (2012) 1091-1100. |
| [17] | B. Das, K. Venkateswarlu, H. Holla, M. Krishnaiah, Sulfonic acid functionalized silica: a remarkably efficient heterogeneous reusable catalyst for α-monobromination of carbonyl compounds using N-bromosuccinimide, J. Mol. Catal. 253 (2006) 107-111. |
| [18] | H. Jin, Z.D. Huang, C.X. Kuang, X.K. Wang, Iron-catalyzed bromination of aryl azides by N-bromosuccinimide: efficient method for the synthesis of brominated aryl azides, Chin. Chem. Lett. 22 (2011) 310-313. |
| [19] | H.M. Meshram, P.N. Reddy, K. Vishnu, K. Sadashiv, J.S. Yadav, A green approach for efficientα-halogenation of β-dicarbonyl compounds and cyclic ketones using N-halosuccinimides inionicliquids, Tetrahedron Lett. 47 (2006) 991-995. |
| [20] | S.S. Arbuj, S.B. Waghmode, A.V. Ramaswamy, Photochemical α-bromination of ketones using N-bromosuccinimide: a simple, mild and efficient method, Tetrahedron Lett. 48 (2007) 1411-1415. |
| [21] | M.V. Adhikari, S.D. Samant, Sonochemical bromination of acetophenones using p-toluenesulfonic acid -N-bromosuccinimide, Ultrason. Sonochem. 9 (2002) 107-111. |
| [22] | I. Pravst, M. Zupan, S. Stavber, Halogenation of ketones with N-halosuccinimides under solvent-free reaction conditions, Tetrahedron 64 (2008) 5191-5199. |
| [23] | M. Hashmat Ali, M. McDermott, Oxidation of thiols to disfiuldes with molecular bromine on hydrated silica gel support, Tetrahedron Lett. 43 (2002) 6271-6273. |
| [24] | E. Selcuk, S. Mehmet Emin, S. Ertan, D. Arif, Bromination of 2,3-dihydrobenzobarrelene and synthesis of its mono-and dibromide derivatives: unexpected Wagner-Meerwein rearrangement on silica gel, Turk. J. Chem. 35 (2011) 587-598. |
| [25] | R.K. Sharma, C. Sharma, Oxidative bromination reaction using Cu2+-perfluorophthalocyanine-immobilized silica gel catalyst under mild reaction conditions, Tetrahedron Lett. 51 (2010) 4415-4418. |
| [26] | K. Smith, M. James, A.G. Mistry, M.R. Bye, D.J. Faulkner, A new method for bromination and polybromination of carbazoles, β-carbolines and iminodibenzyls by using N-bromosuccinimide and silica gel, Tetrahedron 48 (1992) 7479-7488. |
| [27] | H. Alinezhad, M. Tajbakhsh, M. Zare, One-pot regioselective synthesis of 4-bromopyrazole derivatives under solvent free conditions, J. Mex. Chem. Soc. 55 (2011) 238-241. |
| [28] | R.B. Mohan, N.C. Gangi Reddy, Regioselective α-bromination of aralkylketones using N-bromosuccinimide in presence of Montmorillonite K-10 clay: a simple and efficient method, Synth. Commun. 43 (2013) 2603-2614. |
| [29] | A. Tsuruoka, Y. Kaku, H. Kakinuma, et al., Synthesis and antifungal activity of novel thiazole-containing triazole antifungals. II. Optically active ER-30346 and its derivatives, Chem. Pharm. Bull. 46 (1998) 623-630. |
| [30] | W. Peter, B. Joachim, Terpenes and terpene derivatives, synthesis and olfactive properties of (-)-and rac-silphiperfol-5-en-3-ol and of some tris-nor derivatives, Liebigs Ann. Chem. 1992 (1992) 669-678. |
| [31] | R.D. Patil, G. Joshi, S. Adimurthy, B.C. Ranu, Facile one-pot synthesis of abromoketones from olefins using bromide/bromate couple as a nonhazardous brominating agent, Tetrahedron Lett. 50 (2009) 2529-2532. |